Aspectos gonadais, perfil de testosterona e estradiol da tainha Lebranche Mugil liza submetida a diferentes temperaturas durante a fase de larvicultura
DOI:
https://doi.org/10.1590/1809-6891v26e-78854EResumo
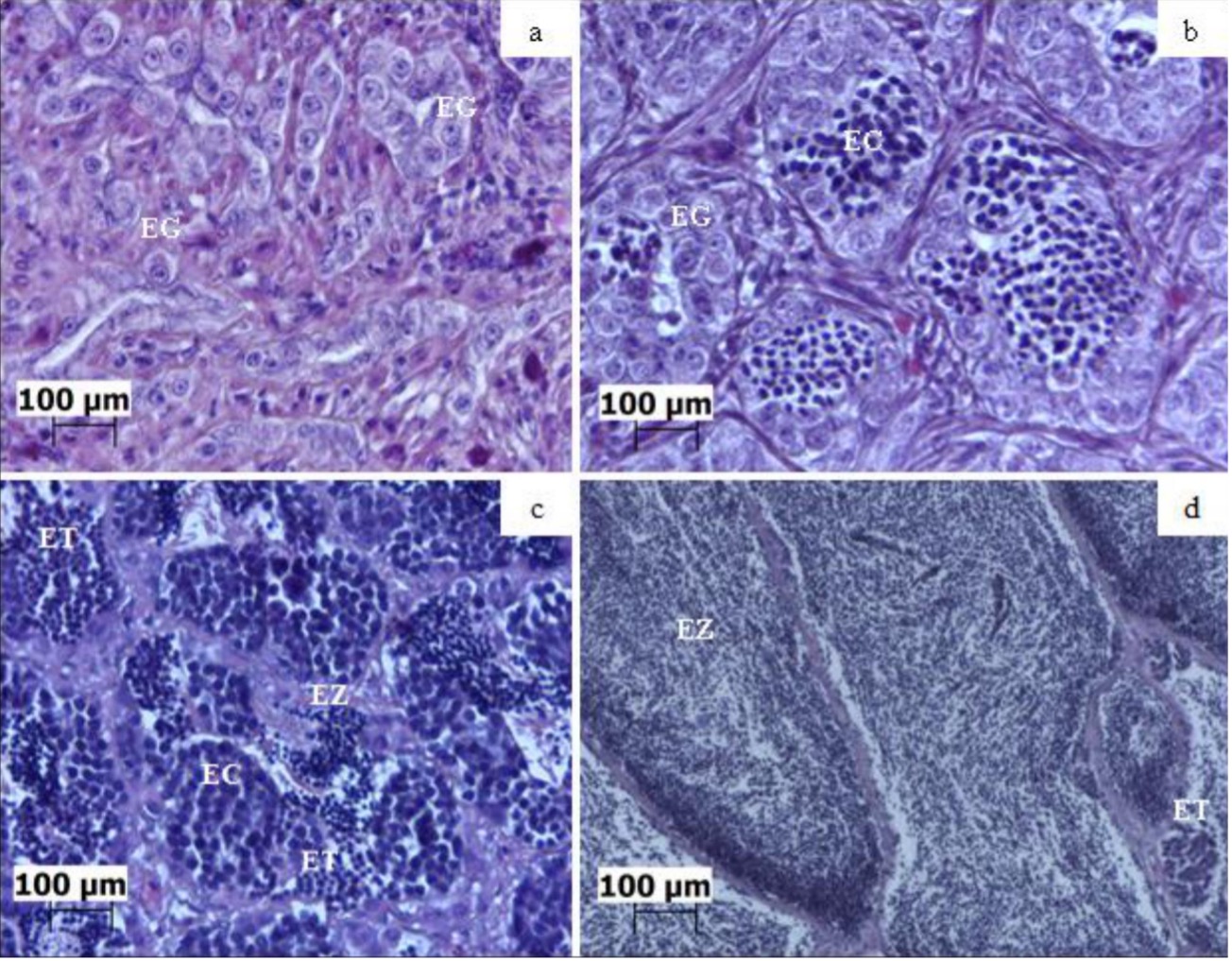
No Brasil, a tainha Lebranche é a espécie Mugilidae mais importante para a indústria pesqueira. Contudo, recentemente a espécie foi recomendada para classificação como quase ameaçada. Neste sentido, a aquicultura surge como uma ferramenta poderosa para a conservação e desenvolvimento dos recursos naturais. O presente estudo avaliou a influência de diferentes temperaturas durante a larvicultura de Mugil liza e seus efeitos tardios no desenvolvimento gonadal e na quantificação hormonal após 24 meses. Inicialmente, os ovos fecundados (45 ovos L-1) foram mantidos em tanque circular de 60 L até a eclosão. Após a eclosão, as larvas foram submetidas a quatro tratamentos em triplicata em diferentes temperaturas (21, 24, 27 e 30 °C) durante 35 dias. Em seguida, os juvenis foram transferidos para unidades de crescimento onde permaneceram identificados, conforme os tratamentos, durante 24 meses. O peso e o comprimento dos peixes foram significativamente diferentes na fase de larvicultura. As análises histológicas mostraram gônadas masculinas imaturas, em maturação e maduras. Os níveis de estradiol nos peixes foram baixos, independente do estágio de maturação. Para a testosterona os maiores valores foram observados em machos maduros (1,29 ± 0,07 ng mL-1). Não foram observadas diferenças significativas (p > 0,05) nas concentrações de estradiol e testosterona em relação à temperatura. Porém, houve diferenças significativas (p < 0,05) na concentração de testosterona dependendo da maturação sexual. Os resultados sugerem que a temperatura exerce um efeito masculinizante na tainha Lebranche. Além disso, a temperatura afetou diretamente o crescimento larval. Em suma, os resultados fornecem evidências de que a temperatura pode desempenhar um papel fundamental na determinação do sexo em M. liza.
Downloads
Referências
Costa MR, Martins RRM, Tomás ARG, Tubino RDA, Monteiro-Neto C. Biological aspects of Mugil liza Valenciennes, 1836 in a tropical estuarine bay in the southwestern Atlantic. Reg Stud Mar Sci. 2021; 43, 101651. https://doi.org/10.1016/j.rsma.2021.101651
Schroeder R, Avigliano E, Volpedo AV, Fortunato RC, Barrulas P, Daros FA, Schwingel PR, Dias MC, Correia AT. Lebranche mullet Mugil liza population structure and connectivity patterns in the southwest Altantic ocean using a multidisciplinary approach. Estuar Coast Shelf Sci. 2023; 288, 108368. https://doi.org/10.1016/j.ecss.2023.108368
Aguirre-Pabon JC, Berdugo GO, Narváez JC. Population structure and low genetic diversity in the threatened lebranche Mugil liza in the Colombian Caribbean. Fish Res. 2022; 256, 106485. https://doi.org/10.1016/j.fishres.2022.106485
Lemos VM, Varela Jr AS, Schwingel PR, Muelbert JH, Vieira JP. Migration and reproductive biology of Mugil liza (Teleostei: Mugilidae) in south Brazil. J Fish Biol. 2014; 85(3), 671-687. https://doi.org/10.1111/jfb.12452
MPA - Ministério da Pesca e Aquicultura. Plano de manejo para o uso sustentável da tainha, Mugil liza Valenciennes, 1836, no Sudeste e Sul do Brasil. 2015. Available in: https://repositorio.icmbio.gov.br/handle/cecav/1514
SAP - Secretaria de Aquicultura e Pesca - Ministério da Agricultura, Pecuária e Abastecimento. GTT - COTA: Relatório do grupo técnico de trabalho para avaliação das cotas de tainha para a temporada de pesca de 2021. 2021; 60 p. Available in: https://www.gov.br/mpa/pt-br/assuntos/pesca/principais-recursos-pesqueiros/tainha
ICMBio - Chico Mendes Institute for Biodiversity Conservation. Red Book of Brazilian Fauna Threatened by Extinction: Volume I. 2018; 1st ed. Ministry of the Environment, Brasília. Available at: https://www.gov.br/icmbio/pt-br/centrais-de-conteudo/publicacoes/publicacoes-diversas/livro-vermelho
Okamoto MH, Sampaio LAND, Maçada ADP. Efeito da temperatura sobre o crescimento e a sobrevivência de juvenis da tainha Mugil platanus Günther, 1880. Atlântica. 2006; 28(1): 61-66. Available in: http://repositorio.furg.br/handle/1/693
Carvalho CVAD, Bianchini A, Tesser MB, Sampaio LA. The effect of protein levels on growth, postprandial excretion and tryptic activity of juvenile mullet Mugil platanus (Günther). Aquac Res. 2010; 41(4), 511-518. https://doi.org/10.1111/j.1365-2109.2009.02340.x
Lisboa V, Barcarolli IF, Sampaio LA, Bianchini A. Effect of salinity on survival, growth and biochemical parameters in juvenile Lebranche mullet Mugil liza (Perciformes: Mugilidae). Neotrop Ichthyol. 2015; 13, 447-452. https://doi.org/10.1590/1982-0224-20140122
Castro J, Magnotti C, Angelo M, Sterzelecki F, Pedrotti F, Oliveira MF, Soligo T, Fracalossi D, Cerqueira VR. Effect of ascorbic acid supplementation on zootechnical performance, haematological parameters and sperm quality of lebranche mullet Mugil liza. Aquac Res. 2019; 50(11), 3267-3274. https://doi.org/10.1111/are.14284
Silva ECD, Sterzelecki FC, Musialak LA, Sugai JK, Castro JDJP, Pedrotti FS, Magnotti C, Cipriano FDS, Cerqueira VR. Effect of feeding frequency on growth performance, blood metabolites, proximate composition and digestive enzymes of Lebranche mullet (Mugil liza) juveniles. Aquac Res. 2020; 51(3), 1162-1169. https://doi.org/10.1111/are.14466
Angelo M, Lisboa MK, Magnotti CCF, Pilotto MR, Mattos JJ, Cerqueira VR. Temperature influence on the embryogenesis, survival and initial development of Mugil liza larvae. Aquac Res. 2021; 52(8), 3705-3712. https://doi.org/10.1111/are.15215
Mylonas CC, Fostier A, Zanuy S. Broodstock management and hormonal manipulations of fish reproduction. Gen Comp Endocrinol. 2010; 165(3), 516-534. https://doi.org/10.1016/j.ygcen.2009.03.007
Baroiller JF, D'Cotta H. Environment and sex determination in farmed fish. Comparative Biochemistry and Physiology Part C: Toxicol Pharmacol. 2001; 130(4), 399-409. https://doi.org/10.1016/S1532-0456(01)00267-8
Strüssmann CA, Nakamura M. Morphology, endocrinology, and environmental modulation of gonadal sex differentiation in teleost fishes. Fish Physiol Biochem. 2002; 26, 13-29. https://doi.org/10.1023/A:1023343023556
Blázquez M, Zanuy S, Carillo M, Piferrer F. Effects of rearing temperature on sex differentiation in the European sea bass (Dicentrarchus labrax L.). J Exp Zool. 1998; 281(3), 207-216. https://doi.org/10.1002/(SICI)1097-010X(19980615)281:3<207::AID-JEZ6>3.0.CO;2-R
Blázquez M. Critical period of androgeninducible sex differentiation in a teleost fish, the European sea bass. J Fish Biol. 2001; 58(2), 342-358. https://doi.org/10.1111/j.1095-8649.2001.tb02257.x
Piferrer F. Endocrine sex control strategies for the feminization of teleost fish. Aquaculture. 2001; 197(1-4), 229-281. https://doi.org/10.1016/S0044-8486(01)00589-0
Jonsson B, Jonsson N. Phenotypic plasticity and epigenetics of fish: embryo temperature affects later-developing lift-history traits. Aquat Biol. 2019; 28, 21-32. https://doi.org/10.3354/ab00707
Vieira JP, Scalabrin C. Migração reprodutiva da “tainha” (Mugil platanus Gunther, 1980) no sul do Brasil. Atlântica. 1991; 13(1), 131-141.
Herbst DF, Hanazaki N. Local ecological knowledge of fishers about the life cycle and temporal patterns in the migration of mullet (Mugil liza) in Southern Brazil. Neotrop Ichthyol. 2014; 12, 879-890. https://doi.org/10.1590/1982-0224-20130156
Ospina-Alvarez N, Piferrer F. Temperature-dependent sex determination in fish revisited: prevalence, a single sex ratio response pattern, and possible effects of climate change. PloS one. 2008; 3(7), e2837. https://doi.org/10.1371/journal.pone.0002837
Cerqueira VR, Carvalho CVAD, Sanches EG, Passini G, Baloi M, Rodrigues RV. Manejo de reprodutores e controle da reprodução de peixes marinhos da costa brasileira. Revista Brasileira de Reprodução Animal. 2017; 41(1), 94-102. Retrieved from: http://cbra.org.br/portal/downloads/publicacoes/rbra/v41/n1/p094-102%20(RB677).pdf
Magnotti C, Santos FC, Pedrotti FS, Cerqueira VR. Advances in reproduction of the lebranche mullet Mugil liza: maturation and spawning of f1 breeders in captivity. Bol Inst Pesca. 2020; 46(3). https://doi.org/10.20950/1678-2305.2020.46.3.586
Yousif OM, Fatah AA, Krishna Kumar K, Minh DV, Hung BV. Induced spawning and larviculture of grey mullet, Mugil cephalus (Linnaeus 1758) in the Emirate of Abu Dhabi. Aquac Asia. 2010; 15(1), 41-43. https://library.enaca.org/AquacultureAsia/Articles/jan-march-2010/10-mullet-larviculture.pdf
Albieri RJ, Araújo FG. Reproductive biology of the mullet Mugil liza (Teleostei: Mugilidae) in a tropical Brazilian bay. Zoologia. 2010; 27, 331-340. https://doi.org/10.1590/S1984-46702010000300003
Vazzoler AE. Biologia da reprodução de peixes teleósteos: teoria e prática (Ed): Anna Emília Amato de Moraes Vazzoler. Maringá: EDUEM; São Paulo: SBI. 1996; 169p. http://old.periodicos.uem.br/~eduem/novapagina/?q=system/files/Biologia%20da%20reprodu%C3%A7%C3%A3o%20de%20peixes%20tele%C3%B3steos.pdf
Chang CF, Lan SC, Chou HY. Gonadal histology and plasma sex steroids during sex differentiation in grey mullet, Mugil cephalus. J Exp Zool. 1995; 272(5), 395-406. https://doi.org/10.1002/jez.1402720509
Magnotti C, Figueroa E, Farias JG, Merino O, Valdebenito I, Oliveira RPS, Cerqueira V. Sperm characteristics of wild and captive lebranche mullet Mugil liza (Valenciennes, 1836), subjected to sperm activation in different pH and salinity conditions. Anim Reprod Sci. 2018; 192, 164-170. https://doi.org/10.1016/j.anireprosci.2018.03.004
Carvalho CVAD, Passini G, Sterzelecki FC, Baloi MF, Cerqueira VR. Maturação, desova e larvicultura da tainha Mugil liza em laboratório. Revista Brasileira de Reprodução Animal. 2019; 43(1), 31-36. http://www.cbra.org.br/portal/downloads/publicacoes/rbra/v43/n1/p31-36%20(RB738).pdf
Crosetti D, Blaber SJ. Biology, ecology and culture of grey mullets (Mugilidae). CRC Press. 2015; pp 539. https://doi.org/10.1201/b19927
González-Castro M, Minos G. Sexuality and reproduction of Mugilidae. In: Crosetti, D., & Blaber, S. J. Biology, ecology and culture of grey mullets (Mugilidae), Taylor & Francis Group, Boca Raton, London, New York. 2016; 539, 227-263.
Pavlidis M, Koumoundouros G, Sterioti A, Somarakis S, Divanach P, Kentouri M. Evidence of temperature‐dependent sex determination in the European sea bass (Dicentrarchus labrax L.). J Exp Zool. 2000; 287(3), 225-232. https://doi.org/10.1002/1097-010X(20000801)287:3%3C225::AID-JEZ4%3E3.0.CO;2-D
Navarro-Martín L, Blázquez M, Viñas J, Joly S, Piferrer F. Balancing the effects of rearing at low temperature during early development on sex ratios, growth and maturation in the European sea bass (Dicentrarchus labrax): limitations and opportunities for the production of highly female-biased stocks. Aquaculture. 2009; 296(3-4), 347-358. https://doi.org/10.1016/j.aquaculture.2009.07.022
Strüssmann CA, Calsina Cota JC, Phonlor G, Higuchi H, Takashima F. Temperature effects on sex differentiation of two South American atherinids, Odontesthes argentinensis and Patagonina hatcheri. Environ Biol Fish. 1996; 47, 143-154. https://doi.org/10.1007/BF00005037
Fessehaye Y, Bovenhuis H, Rezk MA, Crooijmans R, van Arendonk JA, Komen H. Effects of relatedness and inbreeding on reproductive success of Nile tilapia (Oreochromis niloticus). Aquaculture. 2009; 294(3-4), 180-186. https://doi.org/10.1016/j.aquaculture.2009.06.001
Piferrer F. Determinación y diferenciación sexual en los peces. In: La Reproducción de los peces: aspectos básicos y sus aplicaciones en acuicultura. Ed: Carrilo, M. A. Fundación OESA. 2009; 4, p. 249-336. Available in: http://hdl.handle.net/10261/102818. Accessed in 09 January 2024.
Geffroy B, Wedekind C (2020) Effects of global warming on sex ratios in fishes. J Fish Biol. 2020; 97(3), 596-606. https://doi.org/10.1111/jfb.14429
Budd AM, Banh QQ, Domingos JA, Jerry DR. Sex control in fish: approaches, challenges and opportunities for aquaculture. J Mar Sci Eng. 2015; 3(2), 329-355. https://doi.org/10.3390/jmse3020329
Garbin T, Castello JP, Kinas PG. Age, growth, and mortality of the mullet Mugil liza in Brazil's southern and southeastern coastal regions. Fish Res. 2014; 149, 61-68. https://doi.org/10.1016/j.fishres.2013.09.008
McDonough CJ, Roumillat WA, Wenner CA. Sexual differentiation and gonad development in striped mullet (Mugil cephalus L.) from South Carolina estuaries. Fish Bull. 2005; 103(4), 601-619. http://hdl.handle.net/10827/10565
Billard R, Fostier A, Weil C, Breton B. Endocrine control of spermatogenesis in teleost fish. Can J Fish Aquat Sci. 1982; 39(1), 65-79. https://doi.org/10.1139/f82-009
Golshan M, Alavi SMH. Androgen signaling in male fishes: Examples of anti-androgenic chemicals that cause reproductive disorders. Theriogenology. 2019; 139, 58-71. https://doi.org/10.1016/j.theriogenology.2019.07.020
Kumar P, Arasu ART, Kailasamm M, Sukumarran K, Subburj R, Tyagraj G, Natarajan M. Gonadal development and steroid hormone profile of wild caught grey mullet (Mugil cephalus). Biol Rhythm Res. 2015; 46(4), 601-610. https://doi.org/10.1080/09291016.2015.1034974
Downloads
Publicado
Como Citar
Edição
Seção
Licença
Copyright (c) 2025 Ciência Animal Brasileira / Brazilian Animal Science

Este trabalho está licenciado sob uma licença Creative Commons Attribution 4.0 International License.
Autores que publicam nesta revista concordam com os seguintes termos:
- Autores mantém os direitos autorais e concedem à revista o direito de primeira publicação, com o trabalho simultaneamente licenciado sob a Licença Creative Commons Attribution que permite o compartilhamento do trabalho com reconhecimento da autoria e publicação inicial nesta revista.
- Autores têm autorização para assumir contratos adicionais separadamente, para distribuição não-exclusiva da versão do trabalho publicada nesta revista (ex.: publicar em repositório institucional ou como capítulo de livro), com reconhecimento de autoria e publicação inicial nesta revista.
- Autores têm permissão e são estimulados a publicar e distribuir seu trabalho online (ex.: em repositórios institucionais ou na sua página pessoal) a qualquer ponto antes ou durante o processo editorial, já que isso pode gerar alterações produtivas, bem como aumentar o impacto e a citação do trabalho publicado (Veja O Efeito do Acesso Livre).
Declaração de dados
-
Os dados de pesquisa estão disponíveis sob demanda, condição justificada no manuscrito






























