Porcine Circovirus Type 2 Genotypes and PCV3 in Swine Clinical Samples From Brazil
DOI:
https://doi.org/10.1590/1809-6891v25e-77826EAbstract
Porcine circovirus type 2 (PCV2), an important pathogen in swine, causes PCV disease
(PCVD). Although PCVD is effectively controlled using commercial vaccines, its clinical presentation
is changing. Moreover, PCV2 is genetically evolving, with new genotypes emerging in vaccinated or
unvaccinated pigs. In this study, we aimed to verify the presence of the PCV2a, PCV2b, and PCV2d
genotypes in PCV-positive porcine samples. Furthermore, to identify coinfections between the PCV2
genotypes and/or PCV3, which can also induce disease in pigs, we employed a quick, effective, and
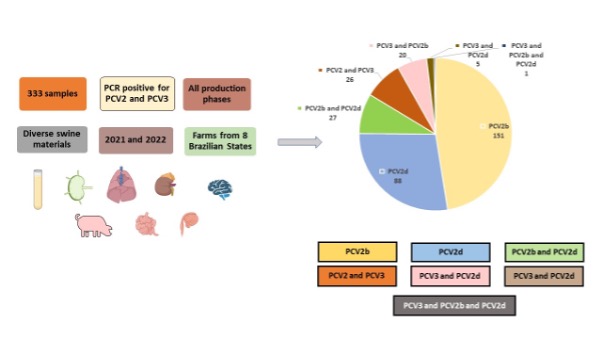
low-cost PCR diagnostic test. In this study, 333 PCV2 PCR and clinically positive samples from various
production stages and herds across Brazil were analyzed. Among these, 266 samples were genotyped,
with PCV2b emerging as the most predominant genotype (56.77% of the positive samples), mainly
observed in nursery pigs. PCV2d was also identified in 33.10% of the samples, primarily from finishing
pigs and breeding sows. The employed PCR test was compared with a commercial kit, proving effective
in PCV2 genotyping. This study demonstrates the significance of PCV2 genotyping, showing PCV2b as
the most predominant genotype responsible for disease in pig farms in Brazil. PCV2a, the prevalent
genotype used in commercial vaccines, was not detected in any of the analyzed samples. While pigs
infected with other PCV2 genotypes may receive some heterologous protection from PCV2a vaccines,
adequate diagnosis and vaccine monitoring for updates must be considered.
Downloads
References
Allan G, McNeilly F, Cassidy J, Reilly G, Adair B, Ellis W, et al. Pathogenesis of porcine circovirus; experimental infections of colostrum deprived piglets and examination of pig foetal material. Veterinary microbiology. 1995;44(1):49-64. https://doi.org/10.1016/0378-1135(94)00136-K
Ellis J, Hassard L, Clark E, Harding J, Allan G, Willson P, et al. Isolation of circovirus from lesions of pigs with postweaning multisystemic wasting syndrome. The Canadian veterinary journal. 1998;39(1):44. canvetj00147-0046.pdf (nih.gov)
Opriessnig T, Meng XJ, Halbur PG. Porcine circovirus type 2 associated disease: update on current terminology, clinical manifestations, pathogenesis, diagnosis, and intervention strategies. J Vet Diagn Invest. 2007;19(6):591-615. https://doi.org/10.1177/104063870701900601
Segalés J, Allan GM, Domingo M. Circoviruses. Diseases of swine. 2019:473-87. https://doi.org/10.1002/9781119350927.ch30
Ciacci-Zanella J, Mores N. Diagnosis of post-weaning multisystemic wasting syndrome in pigs in Brazil caused by porcine circovirus type 2. Arquivo Brasileiro De Medicina Veterinaria E Zootecnia. 2003;55(5):522-7. https://doi.org/10.1590/s0102-09352003000500002
Silva FMFd, Silva Júnior A, Peternelli EFdO, Viana VW, Chiarelli Neto O, Fietto JLR, et al. Retrospective study on Porcine circovirus-2 by nested pcr and real time pcr in archived tissues from 1978 in brazil. Brazilian Journal of Microbiology. 2011;42:1156-60. https://doi.org/10.1590/s1517-83822011000300039
Zanella JRC, Morés N, Barcellos DESNd. Main endemic health threats in the swine production chain in Brazil. Pesquisa Agropecuária Brasileira. 2016;51(5):443-53. https://doi.org/10.1590/s0100-204x2016000500004
Opriessnig T, Meng X-J, Halbur PG. Porcine circovirus type 2–associated disease: update on current terminology, clinical manifestations, pathogenesis, diagnosis, and intervention strategies. Journal of Veterinary Diagnostic Investigation. 2007;19(6):591-615. https://doi.org/10.1177/104063870701900601
Carman S, Cai HY, DeLay J, Youssef SA, McEwen BJ, Gagnon CA, et al. The emergence of a new strain of porcine circovirus-2 in Ontario and Quebec swine and its association with severe porcine circovirus associated disease—2004–2006. Canadian Journal of Veterinary Research. 2008;72(3):259. https://doi.org/10.1136/vr.167.7.260
Xiao CT, Halbur PG, Opriessnig T. Complete genome sequence of a novel porcine circovirus type 2b variant present in cases of vaccine failures in the United States. J Virol. 2012;86(22):12469. https://doi.org/10.1128/jvi.02345-12
Yao J, Qin Y, Zeng Y, Ouyang K, Chen Y, Huang W, et al. Genetic analysis of porcine circovirus type 2 (PCV2) strains between 2002 and 2016 reveals PCV2 mutant predominating in porcine population in Guangxi, China. BMC veterinary research. 2019;15:1-11. https://doi.org/10.1186/s12917-019-1859-z
Opriessnig T, O’Neill K, Gerber PF, de Castro AM, Gimenéz-Lirola LG, Beach NM, et al. A PCV2 vaccine based on genotype 2b is more effective than a 2a-based vaccine to protect against PCV2b or combined PCV2a/2b viremia in pigs with concurrent PCV2, PRRSV and PPV infection. Vaccine. 2013;31(3):487-94. https://doi.org/10.1016/j.vaccine.2012.11.030
Gava D, Serrao VHB, Fernandes LT, Cantao ME, Ciacci-Zanella JR, Mores N, et al. Structure analysis of capsid protein of Porcine circovirus type 2 from pigs with systemic disease. Braz J Microbiol. 2018;49(2):351-7. https://doi.org/10.1016/j.bjm.2017.08.007
Franzo G, Segalés J. Porcine circovirus 2 (PCV-2) genotype update and proposal of a new genotyping methodology. PLoS One. 2018;13(12):e0208585. https://doi.org/10.1371/journal.pone.0208585
Lv Q, Wang T, Deng J, Chen Y, Yan Q, Wang D, et al. Genomic analysis of porcine circovirus type 2 from southern China. Vet Med Sci. 2020;6(4):875-89. https://doi.org/10.1002/vms3.288
Wang Y, Noll L, Lu N, Porter E, Stoy C, Zheng W, et al. Genetic diversity and prevalence of porcine circovirus type 3 (PCV3) and type 2 (PCV2) in the Midwest of the USA during 2016-2018. Transbound Emerg Dis. 2020;67(3):1284-94. https://doi.org/10.1111/tbed.13467
Opriessnig T, Karuppannan AK, Castro AM, Xiao C-T. Porcine circoviruses: Current status, knowledge gaps and challenges. Virus research. 2020;286:198044. https://doi.org/10.1016/j.virusres.2020.198044
Assao VS, Santos MR, Rosado NCL, Bressan GC, Fietto JLR, Chang YF, et al. Genetic diversity of porcine circovirus 3 strains and the first detection of two different PCV3 strains coinfecting the same host in Minas Gerais, Brazil. Arch Virol. 2021;166(5):1463-8. https://doi.org/10.1007/s00705-021-05032-y
Rodrigues ILF, Cruz ACM, Souza AE, Knackfuss FB, Costa CHC, Silveira RL, et al. Retrospective study of porcine circovirus 3 (PCV3) in swine tissue from Brazil (1967-2018). Braz J Microbiol. 2020;51(3):1391-7. https://doi.org/10.1007/s42770-020-00281-6
ABPA. Brazilian Association of Animal Protein - Annual Report - 2023. 2023 [Available from: https://abpa-br.org/wp-content/uploads/2023/04/ABPA.-Annual-Report-2023..pdf.
EMBRAPA. CIAS - Central de Inteligencia de Aves e Suinos Brazil: Embrapa Swine and Poultry; 2023 [Available from: https://www.embrapa.br/en/suinos-e-aves/cias/estatisticas.
CEDISA. Centro de Diagnostico em Sanidade Animal 2023 [Available from: http://www.cedisa.org.br/home/.
Sibila M, Rocco C, Franzo G, Huerta E, Domingo M, Nunez JI, et al. Genotyping of Porcine Circovirus 2 (PCV-2) in Vaccinated Pigs Suffering from PCV-2-Systemic Disease between 2009 and 2020 in Spain. Pathogens. 2021;10(8). https://doi.org/10.3390/pathogens10081016
Dupont K, Nielsen E, Baekbo P, Larsen L. Genomic analysis of PCV2 isolates from Danish archives and a current PMWS case–control study supports a shift in genotypes with time. Veterinary microbiology. 2008;128(1-2):56-64. https://doi.org/10.1016/j.vetmic.2007.09.016
Hesse R, Kerrigan M, Rowland RR. Evidence for recombination between PCV2a and PCV2b in the field. Virus research. 2008;132(1-2):201-7. https://doi.org/10.1016/j.virusres.2007.10.013
Kim S-C, Nazki S, Kwon S, Juhng J-H, Mun K-H, Jeon D-Y, et al. The prevalence and genetic characteristics of porcine circovirus type 2 and 3 in Korea. BMC veterinary research. 2018;14:1-9. https://doi.org/10.1186/s12917-018-1614-x
Kwon T, Lee D-U, Yoo SJ, Sang HJ, Shin JY, Lyoo YS. Genotypic diversity of porcine circovirus type 2 (PCV2) and genotype shift to PCV2d in Korean pig population. Virus Research. 2017;228:24-9. https://doi.org/10.1016/j.virusres.2016.11.015
Mone NK, Clark NJ, Kyaw-Tanner M, Turni C, Barnes TS, Parke CR, et al. Genetic analysis of porcine circovirus type 2 (PCV2) in Queensland, Australia. Aust Vet J. 2020;98(8):388-95. https://doi.org/10.1111/avj.12952
Gagnon CA, Tremblay D, Tijssen P, Venne M-H, Houde A, Elahi SM. The emergence of porcine circovirus 2b genotype (PCV-2b) in swine in Canada. The Canadian Veterinary Journal. 2007;48(8):811. https://doi.org/10.1016/j.vetmic.2009.09.072
Franzo G, Segales J. Porcine Circovirus 2 Genotypes, Immunity and Vaccines: Multiple Genotypes but One Single Serotype. Pathogens. 2020;9(12). https://doi.org/10.3390/pathogens9121049
Ouyang T, Zhang X, Liu X, Ren L. Co-infection of swine with porcine circovirus type 2 and other swine viruses. Viruses. 2019;11(2):185. https://doi.org/10.3390/v11020185
Park KH, Chae C. The prevalence of porcine circovirus type 2e (PCV2e) in Korean slaughter pig lymph nodes when compared with other PCV2 genotypes. Transbound Emerg Dis. 2021;68(6):3043-7. https://doi.org/10.1111/tbed.13975
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Brazilian Animal Science/ Ciência Animal Brasileira

This work is licensed under a Creative Commons Attribution 4.0 International License.
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.
- Authors are permitted and encouraged to post their work online (e.g. in institutional repositories or on their website) prior to and during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See The Effect of Open Access).































