Evaluation of the corneal epithelium of rabbits treated with preservative-free eye drops containing ketorolac tromethamine or diclofenac sodium
DOI:
https://doi.org/10.1590/1809-6891v24e-75047EAbstract
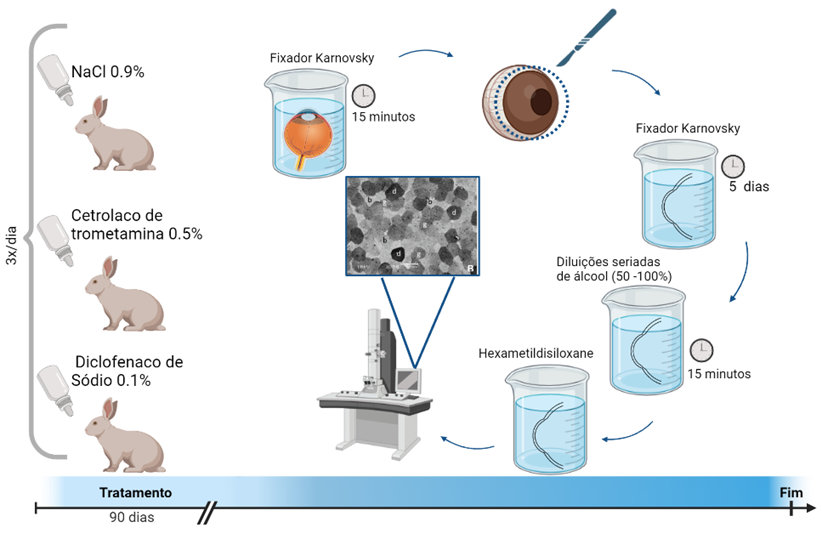
This study aimed to evaluate the corneal epitheliotoxic effects of preservative-free ketorolac tromethamine 0.5% and diclofenac sodium 0.1% eye drops in rabbits. Seventeen New Zealand rabbits were randomly divided into three groups: the 0.5% ketorolac tromethamine group, the 0.1% diclofenac sodium group, and the control group (0.9% NaCl). For each rabbit, both eyes were treated three times daily according to their treatment group. The corneal epithelia were analyzed using scanning electron microscopy to observe the number of light, grey, and dark cells; the number of epithelial holes; and the loss of hexagonal shape. Both of the formulations administered caused changes in the healthy corneal epithelia of rabbits. Except for number of epithelial holes (p < 0.05), all the parameters showed a statistically significant difference between the groups. The number of dark cells was highest in the ketorolac tromethamine group (p<0.05). The number of grey cells was higher in the diclofenac sodium group than in the control group (p =0.003). A higher number of dark cells was associated with a smaller number of light cells (r =-0.577, p < 0.001). Loss of shape showed a direct correlation with the number of dark cells (r=0.524, p=0.002). Based on the results presented, it was possible to conclude that ketorolac tromethamine 0.5% was more toxic to rabbit corneal epithelium than diclofenac sodium 0.1%.
Keywords: cornea; nonsteroidal anti-inflammatory agent; ophthalmic solutions; scanning electron microscopy
Downloads
References
Flach AJ. Corneal melts associated with topically applied nonsteroidal anti-inflammmatory drugs. Transactions of the American Ophthalmological Society. 2001;(99):205-2. (https://pubmed.ncbi.nlm.nih.gov/11797308/)
Lee JS, Kim YH, Park YM. The Toxicity of nonsteroidal anti-inflammatory eye drops against human corneal epithelial cells in vitro Journal of Korean medical science. 2015;30(12):1856-64. (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4689832/)
Stroobants A, Fabre K, Maudgal PC. Effect of non-steroidal anti-inflammatory drugs (NSAID) on the rabbit corneal epithelium studied by scanning electron microscopy. Bulletin de la Societe belge d'ophtalmologie. 2000;276:73-82. (https://www.ophthalmologia.be/download.php?dof_id=65)
Guidera AC, Luchs JI, Udell IJ. Keratitis, ulceration, and perforation associated with topical nonsteroidal anti-inflammatory drugs. Ophthalmology. 2001;108(5):936-44. (https://www.aaojournal.org/article/S0161-6420(00)00538-8/fulltext)
Kim SJ. Flach AJ, Jampol LM. Nonsteroidal anti-inflammatory drugs in ophthalmology. Survey of ophthalmology 2010;55(2):108-33. (https://pubmed.ncbi.nlm.nih.gov/20159228/)
Shimazaki J, Saito H, Yang HY. Persistent epithelial defect following penetrating keratoplasty: an adverse effect of diclofenac eyedrops. Cornea. 1995;14(6):623-7. (https://journals.lww.com/corneajrnl/Abstract/1995/11000/Persistent_Epithelial_Defect_Following_Penetrating.18.aspx)
Ayaki M, Iwasawa A, Soda M, Yaguchi S, Koide R. Cytotoxicity of five fluoroquinolone and two nonsteroidal anti-inflammatory benzalkonium chloride-free ophthalmic solutions in four corneoconjunctival cell lines. Clinical ophthalmology. 2010;20(4):1019-24. (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2946991/)
Ferraz LCB, Schellini AS, Gregorio EA. Efeito do colírio de 5-fluorouracil sobre o epitélio corneano íntegro de coelhos. Arquivos Brasileiros de Oftalmologia 2003;66(4):493-7. (https://www.scielo.br/j/abo/a/HXxmrgbppG4vchsdTPdwNDx/?lang=pt)
Epstein SP, Ahdoot M, Marcus E, Asbell AP. Comparative toxicity of preservatives on immortalized corneal and conjunctival epithelial cells. Journal of ocular pharmacology and therapeutics : the official journal of the Association for Ocular Pharmacology and Therapeutics. 2009;25(2):113-9. (https://pubmed.ncbi.nlm.nih.gov/19284328/)
Hashemian MN, Rahimi F, Alipour F, Ahmadraji AA, Torabi HR, Tavakoli N, Moghadam RS. The effect of topical diclofenac sodium 0.1% on the corneal epithelial healing after photorefractive keratectomy. Iranian Journal of Ophthalmology. 2011;23(2):27-30. (https://www.sid.ir/paper/599363/en)
Kawahara A, Utsunomiya T, Kato Y, Takayanagi Y. Comparison of effect of nepafenac and diclofenac ophthalmic solutions on cornea, tear film, and ocular surface after cataract surgery: the results of a randomized trial. Clinical ophthalmology. 2016;4(10):385-91. (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4786065/)
Aragona P, Tripodi G, Spinella R, Laganá E, Ferreri G. The effects of the topical administration of non-steroidal anti-inflammatory drugs on corneal epithelium and corneal sensitivity in normal subjects. Eye. 2000;(14):206-10. (https://pubmed.ncbi.nlm.nih.gov/10845018/)
Duan P, Liu Y, Li J. The comparative efficacy and safety of topical non-steroidal anti-inflammatory drugs for the treatment of anterior chamber inflammation after cataract surgery: a systematic review and network meta-analysis. Graefe's archive for clinical and experimental ophthalmology [Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie]. 2017;255(4): 639-49. (https://pubmed.ncbi.nlm.nih.gov/28130595/)
Barba KR, Samy A, Lai C, Perlman JI, Bouchard CS. Effect of topical anti-inflammatory drugs on corneal and limbal wound healing. Journal of cataract and refractive surgery. 2000;26(6):893-7.( https://pubmed.ncbi.nlm.nih.gov/10889437/)
Gaynes BI, Onyekwuluje A. Topical ophthalmic NSAIDs: a discussion with focus on nepafenac ophthalmic suspension. Clinical ophthalmology. 2008;2(2):355-68. (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2693998/)
Wilson DJ, Schutte SM, Abel SR. Comparing the efficacy of ophthalmic NSAIDs in common indications: a literature review to support cost-effective prescribing. The Annals of pharmacotherapy 2015 49(6):727-34. (https://pubmed.ncbi.nlm.nih.gov/25725037/)
Iwamoto S, Koga T, Ohba M, Okuno T, Koike M, Murakami A, et al. Non-steroidal anti-inflammatory drug delays corneal wound healing by reducing production of 12- hydroxyheptadecatrienoic acid, a ligand for leukotriene B4 receptor 2. Scientific Reports. 2017;7(1):13267.( https://www.nature.com/articles/s41598-017-13122-8)
Raizman MB, Hamrah P, Holland EJ, Kim T, Mah FS, Rapuano CJ, Ulrich RG. Drug-induced corneal epithelial changes. Survey of ophthalmology. 2017;62(3):286-301. (https://pubmed.ncbi.nlm.nih.gov/27890620/)
Rigas B, Huang W, Honkanen R. NSAID-induced corneal melt: Clinical importance, pathogenesis, and risk mitigation. Survey of ophthalmology. 2020;65(1):1-11. (https://pubmed.ncbi.nlm.nih.gov/31306671/)
Guo Y, Satpathy M, Wilson G, Srinivas SP. Benzalkonium chloride induces dephosphorylation of myosin light chain in cultured corneal epithelial cells nvestigative ophthalmology & visual science. 2007;48(5):936-44. (https://pubmed.ncbi.nlm.nih.gov/17460253/)
Uematsu M, Mohamed YH, Onizuka N , Ueki R , Inoue D , Fujikawa A. Acute corneal toxicity of latanoprost with different preservatives. Cutaneous and ocular toxicology 2015;35(2):1-6. (https://pubmed.ncbi.nlm.nih.gov/26113030/)
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Brazilian Animal Science/ Ciência Animal Brasileira

This work is licensed under a Creative Commons Attribution 4.0 International License.
Authors who publish with this journal agree to the following terms:
- Authors retain copyright and grant the journal right of first publication with the work simultaneously licensed under a Creative Commons Attribution License that allows others to share the work with an acknowledgement of the work's authorship and initial publication in this journal.
- Authors are able to enter into separate, additional contractual arrangements for the non-exclusive distribution of the journal's published version of the work (e.g., post it to an institutional repository or publish it in a book), with an acknowledgement of its initial publication in this journal.
- Authors are permitted and encouraged to post their work online (e.g. in institutional repositories or on their website) prior to and during the submission process, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See The Effect of Open Access).































