Microscopia especular das diferentes regiões da córnea de suínos - estudo ex vivo
DOI:
https://doi.org/10.1590/1809-6891v24e-75138EResumo
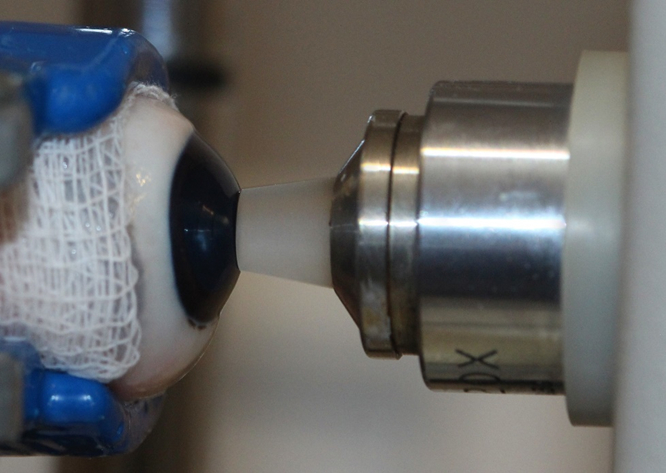
O objetivo do presente estudo foi avaliar a densidade endotelial e a hexagonalidade das células endoteliais nas diferentes regiões da córnea de suínos utilizando a microscopia especular de contato. Foram estudados 24 bulbos oculares de 12 suínos (Sus scrofa domesticus), machos, com seis meses de idade e da raça Large White. A microscopia especular de contato foi realizada nas regiões central, superior, inferior, lateral e medial. A densidade endotelial média na região central foi de 1865 células/mm², na região superior foi de 1877 células/mm², na região inferior foi de 1854 células/mm², na região lateral foi de 1847 células/mm² e na região medial foi de 1831 células/mm². Na região central, a hexagonalidade foi de 53%, na região superior foi de 54%, na região inferior foi de 54%, na região lateral foi de 54%, na região medial foi de 54%. Não foram observadas diferenças significativas na densidade celular e na hexagonalidade nas diferentes regiões da córnea analisadas. Este estudo demonstrou que a densidade endotelial e a hexagonalidade da área central da córnea representam todo o mosaico endotelial.
Palavras-chave: córnea; endotélio; morfologia; contagem celular; suíno.
Downloads
Referências
Tuft SJ, Coster DJ. The corneal endothelium. Eye. 1990;4(3):389-424. Available from: doi:10.1038/eye.1990.53.
Saad HA, Terry MA, Shamie N, Chen ES, Friend DF, Holiman JD et al. An easy and inexpensive method for quantitative analysis of endothelial damage by using vital dye staining and adobe photoshop software. Cornea. 2008;27(7):818-824. Available from: doi:10.1097/ico.0b013e3181705ca2.
Smeringaiova I, Merjava SR, Stranak Z, Studeny P, Bednar J, Jirsova K. Endothelial wound repair of the organ-cultured porcine corneas. Current Eye Research. 2018;43(7):856-865. Available from: doi:10.1080/02713683.2018.1458883.
Pigatto JAT, Andrade MC, Laus JL, Santos JM, Brooks DE, Guedes PM, et al. Morphometric analysis of the corneal endothelium of Yacare caiman (Caiman yacare) using scanning electron microscopy. Veterinary Ophthalmology. 2004;7(3):205-208. Available from: doi:10.1111/j.1463-5224.2004.04025.
Selig B, Vermeer KA, Rieger B, Hillenaar T, Hendriks CLL. Fully automatic evaluation of the corneal endothelium from in vivo confocal microscopy. BMC Medical Imaging. 2015;15(1)1-15. Available from: doi:10.1186/s12880-015-0054-3.
McCarey BE, Edelhauser HF, Lynn MJ. Review of corneal endothelial specular microscopy for FDA clinical trials of refractive procedures, surgical devices, and new intraocular drugs and solutions. Cornea. 2008;27(1):1-16. Available from: doi:10.1097/ico.0b013e31815892da.
Van Schaick W, van Dooren BT, Mulder PG, Völker-Dieben HJ. Validity of endothelial cell analysis methods and recommendations for calibration in Topcon SP-2000P specular microscopy. Cornea. 2005;24(5):538-544. Available from: doi:10.1097/01.ico.0000151505.03824.6c.
Gwin RM, Lerner I, Warren JK, Gum G. Decrease in canine corneal endothelial cell density and increase in corneal thickness as functions of age. Investigative Ophthalmology & Visual Science. 1982;22(2):267-271. Available from: https://pubmed.ncbi.nlm.nih.gov/7056641/.
Pigatto JAT, Abib FC, Pereira GT, Barros PSM, Freire CD, Laus JL. Density of corneal endothelial cells in eyes of dogs using specular microscopy. Brazilian Journal of Veterinary Research and Animal Science. 2006; 43(4): 476-480. Available from: doi: 10.11606/issn.1678-4456.bjvras.2006.26462.
Pigatto JAT, Cerva C, Freire CD, Abib FC, Bellini LP, Barros PSM, et al. Morphological analysis of the corneal endothelium in eyes of dogs using specular microscopy. Pesquisa Veterinária Brasileira. 2008;28(9):427-430. Available from: doi:10.1590/s0100-736x2008000900006.
Chan-Ling T, Curmi J. Changes in corneal endothelial morphology in cats as a function of age. Current Eye Research. 1988;7(4):387-392. Available from: doi:10.3109/02713688809031788.
Franzen AA, Pigatto JAT, Abib FC, Albuquerque L, Laus JL. Use of specular microscopy to determine corneal endothelial cell morphology and morphometry in enucleated cat eyes. Veterinary Ophthalmology. 2010;13(4):222-226. Available from: doi:10.1111/j.1463-5224.2010.00787.x.
Coyo N, Peña MT, Costa D, Ríos J, Lacerda R, Leiva M. Effects of age and breed on corneal thickness, density, and morphology of corneal endothelial cells in enucleated sheep eyes. Veterinary Ophthalmology. 2016;19(5):367-372. Available from: doi:10.1111/vop.1230.
Andrew SE, Ramsey DT, Hauptman JG, Brooks DE. Density of corneal endothelial cells and corneal thickness in eyes of euthanatized horses. American Journal of Veterinary Research. 2001;62(4):479-482. Available from: doi:10.2460/ajvr.2001.62.479.
Andrew SE, Willis AM, Anderson DE. Density of corneal endothelial cells, corneal thickness, and corneal diameters in normal eyes of llamas and alpacas. American Journal of Veterinary Research. 2002;63(3):326-329. Available from: doi:10.2460/ajvr.2002.63.326.
Albuquerque L, Pigatto JAT, Pacicco LVR. Analysis of the corneal endothelium in eyes of chickens using contact specular microscopy. Semina: Ciências Agrárias. 2015; 36(2): 4199-4206. Available from: doi:10.5433/1679-0359.2015v36n6supl2p4199.
Bercht BS, Albuquerque L, Araujo ACP, Pigatto JAT. Specular microscopy to determine corneal endotelial cell mosphology and morphometry in chinchillas (Chinchilla lanigera) in vivo. Veterinary Ophthalmology. 2015; 18(1): 137-142. Available from: doi:10.1111/vop.12236.
Morita H. Specular microscopy of corneal endothelial cells in rabbits. Journal of Veterinary Medical Science. 1995;57(2):273-277. Available from: doi:10.1292/jvms.57.273.
Brambatti G, Albuquerque L, Pigatto JAT, Vargas EDB, Neumann CF. Corneal endothelial cell density and morphology in rabbits’ eyes using contact specular microscopy. Ciência Rural. 2017;47(12):1-5. Available from: doi:10.1590/0103-8478cr20170027.
Coyo N, Leiva M, Costa D, Rios J, Peña T. Corneal thickness, endothelial cell density, and morphological and morphometric features of corneal endothelial cells in goats. American Journal of Veterinary Research. 2018;79(10):1087-1092. Available from: doi:10.2460/ajvr.79.10.1087.
Hashimoto C, Kurosaka D, Uetsuki Y. Teaching continuous curvilinear capsulorhexis using a postmortem pig eye with simulated cataract. Journal of Cataract & Refractive Surgery. 2001;27(6):814-816. Available from: doi:10.1016/s0886-3350(00)00728-8.
Kermani O, Oberheide U. Comparative micromorphologic in vitro porcine study of IntraLase and Femto LDV femtosecond lasers. Journal of Cataract & Refractive Surgery. 2008;34(8):1393-1399. Available from: doi:10.1016/j.jcrs.2008.04.037.
Nicholls S, Bailey M, Mitchard L, Dick A. Can the corneal endothelium of the pig proliferate in vivo? Acta Ophthalmologica. 2009;87:0. Available from: doi:10.1111/j.1755-3768.2009.2271.x.
Heichel J, Wilhelm F, Kunert K, Schlueter R, Stuhltraeger U, Hammer T. Influence of microkeratome parameters on the stromal bed and flap edge quality in laser in situ keratomileusis. Clinical Ophthalmology. 2014;8: 61-69. Available from: doi:10.2147/opth.s51200.
Heichel J, Blum M, Duncker GIW, Sietmann R, Kunert KS. Surface quality of porcine corneal lenticules after Femtosecond Lenticule Extraction. Ophthalmic Research. 2011;46(2):107-112. Available from: doi:10.1159/000323814.
Gros-Otero J, Ketabi S, Cañones-Zafra R, Garcia-Gonzalez M, Parafita-Fernandez A, Villa-Collar C, et al. Analysis of corneal stromal roughness after iFS 150 kHz and LenSx femtosecond LASIK flap creation in porcine eyes. Graefe's Archive for Clinical and Experimental Ophthalmology. 2019;257(12):2665-2670. Available from: doi:10.1007/s00417-019-04497-7.
Hara H, Cooper DK. Xenotransplantation - the future of corneal transplantation? Cornea. 2011;30(4):371-378. Available from: doi:10.1097/ICO.0b013e3181f237ef.
Lee SE, Mehra R, Fujita M, Roh DS, Long C, Lee W, et al. Characterization of porcine corneal endothelium for xenotransplantation. Seminars in Ophthalmology. 2014;29(3):127-135. Available from: doi:10.3109/08820538.2013.787104.
Bahn CF, Glassman RM, MacCallum DK, Lillie JH, Meyer RF, Robinson BJ, et al. Postnatal development of corneal endothelium. Investigative Ophthalmology & Visual Science. 1986;27(1):44-51. Available from: https://pubmed.ncbi.nlm.nih.gov/3941037/.
Tamayo-Arango LJ, Baraldi-Artoni SM, Laus JL, Vicenti FAM, Pigatto JAT, Abib FC. Ultrastructural morphology and morphometry of the normal corneal endothelium of adult crossbred pig. Ciência Rural. 2009;39(1):117-122. Available from: doi:10.1590/s0103-84782009000100018.
Clerot LL, Hünning PS, Bettio M, Torikachvili M, Petersen MB, Silva AF, et al. Morphology of endothelial cells from different regions of the swine cornea. Acta Scientiae Veterinariae. 2019; 47:1-6. Available from: doi.org/10.22456/1679-9216.89436.
Zeng Y, Yang J, Huang K, Lee Z, Lee X. A comparison of biomechanical properties between human and porcine cornea. Journal of Biomechanics. 2001;34(4):533-537. Available from:10.1016/s0021-9290(00)00219-0.
Fujita M, Mehra R, Lee SE, Roh DS, Long C, Funderburgh JL, et al. Comparison of proliferative capacity of genetically-engineered pig and human corneal endothelial cells. Ophthalmic Research. 2013;49(3):127-138. Available from: doi:10.1159/000342978.
Collin SP, Collin HB. A comparative study of the corneal endothelium in vertebrates. Clinical and Experimental Optometry. 1998;81(6):245-254. Available from: doi:10.1111/j.1444-0938.1998.tb06744.x.
Stapleton S, Peiffer RL Jr. Specular microscopic observations of the clinically normal canine corneal endothelium. American Journal of Veterinary Research. 1979;40(12):1803-1804. Available from: https://pubmed.ncbi.nlm.nih.gov/525905/.
Price NC, Cheng H. Contact and noncontact specular microscopy. British Journal of Ophthalmology. 1981;65(8):568-574. Available from: doi:10.1136/bjo.65.8.568.
Abib FC, Holzchuh R, Schaefer A, Schaefer T, Godois R. The endothelial sample size analysis in corneal specular microscopy clinical examinations. Cornea. 2012;31(5):546-550. Available from: doi: 10.1097/ICO.0b013e3181cc7961.
Chaurasia S, Vanathi M. Specular microscopy in clinical practice. Indian Journal of Ophthalmology. 2021;69(3):517-524. Available from: doi:10.4103/ijo.IJO_574_20.
Doughty MJ. Subjective vs. objective analysis of the corneal endothelial cells in the rabbit cornea by scanning electron microscopy - a comparison of two different methods of corneal fixation. Veterinary Ophthalmology. 2006;9(2):127-135. Available from: doi:10.1111/j.1463-5224.2006.00449.x.
Huang J, Maram J, Tepelus TC, Modak C, Marion K, Sadda SR, et al. Comparison of manual & automated analysis methods for corneal endothelial cell density measurements by specular microscopy. Journal of Optometry. 2018;11(3):182-191. Available from: doi:10.1016/j.optom.2017.06.001.
Coyo N, Leiva M, Costa D, Molina R, Nicolás O, Ríos J, et al. Endothelial cell density and characterization of corneal endothelial cells in the Tawny Owl (Strix aluco) using specular microscopy. Veterinary Ophthalmology. 2019;22(2):177-182. Available from: doi:10.1111/vop.12578.
Abib FC, Barreto Junior J. Behavior of corneal endothelial density over a lifetime. Journal of Cataract & Refractive Surgery. 2001;27(10):1574-1578. Available from: doi:10.1016/s0886-335.
Amann J, Holley GP, Lee SB, Edelhauser HF. Increased endothelial cell density in the paracentral and peripheral regions of the human cornea. American Journal of Ophthalmology. 2003;135(5):584-590. Available from: doi:10.1016/s0002-9394(02)02237-7.
Müller A, Craig JP, Grupcheva CN, McGhee CN. The effects of corneal parameters on the assessment of endothelial cell density in the elderly eye. British Journal of Ophthalmology. 2004;88(3):325-330. Available from: doi:10.1136/bjo.2003.019315.
Downloads
Publicado
Como Citar
Edição
Seção
Licença
Copyright (c) 2023 Ciência Animal Brasileira / Brazilian Animal Science

Este trabalho está licenciado sob uma licença Creative Commons Attribution 4.0 International License.
Autores que publicam nesta revista concordam com os seguintes termos:
- Autores mantém os direitos autorais e concedem à revista o direito de primeira publicação, com o trabalho simultaneamente licenciado sob a Licença Creative Commons Attribution que permite o compartilhamento do trabalho com reconhecimento da autoria e publicação inicial nesta revista.
- Autores têm autorização para assumir contratos adicionais separadamente, para distribuição não-exclusiva da versão do trabalho publicada nesta revista (ex.: publicar em repositório institucional ou como capítulo de livro), com reconhecimento de autoria e publicação inicial nesta revista.
- Autores têm permissão e são estimulados a publicar e distribuir seu trabalho online (ex.: em repositórios institucionais ou na sua página pessoal) a qualquer ponto antes ou durante o processo editorial, já que isso pode gerar alterações produtivas, bem como aumentar o impacto e a citação do trabalho publicado (Veja O Efeito do Acesso Livre).






























