Uso potencial de pigmentos bacterianos como drogas anticâncer e toxicidade reprodutiva feminina: uma revisão
DOI:
https://doi.org/10.1590/1809-6891v23e-72911EResumo
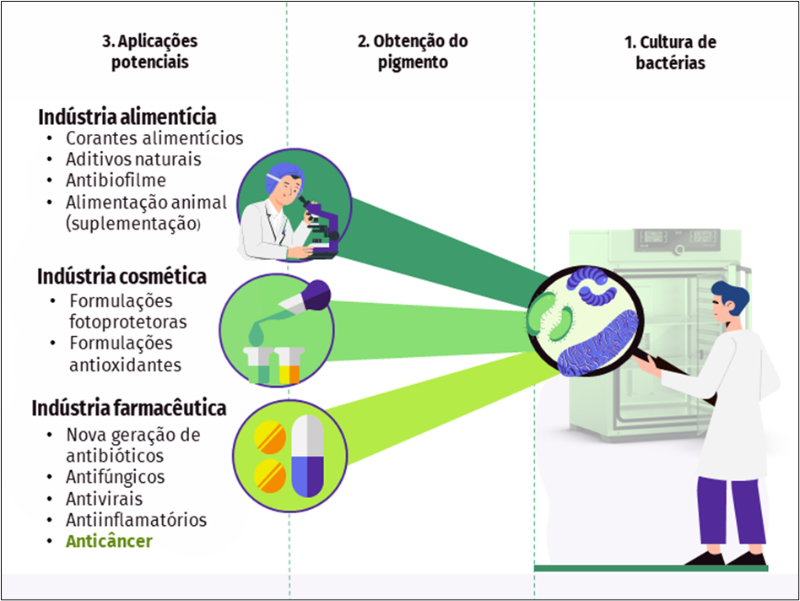
Os compostos bioativos naturais obtidos de microrganismos têm despertado especial interesse da indústria nos últimos anos. Esta atenção ocorre em um momento em que o esgotamento de recursos naturais é pronunciado, e a aquisição de novos insumos e produtos bioativos de origem vegetal representa um desafio para as próximas gerações. Neste sentido, a prospecção para a produção e uso em larga escala dos pigmentos bacterianos tem representado uma importante estratégia para o desenvolvimento de novos produtos. Uma grande variedade de propriedades foi atribuída a estas substâncias, entre elas, o potencial terapêutico contra doenças importantes, como o câncer. Existe um consenso de que os protocolos quimioterápicos disponíveis são conhecidos por afetarem negativamente a fertilidade de pacientes com câncer. Grande parte dos efeitos deletérios da quimioterapia está relacionado à citotoxicidade das drogas usadas para este fim, que além das células cancerosas, afetam as células normais. Nesse sentido, as propriedades naturais atribuídas aos pigmentos bacterianos associadas à baixa citotoxicidade e relevante seletividade, os qualificaram como potenciais drogas anticâncer. No entanto, pouco se tem de informação a respeito da toxicidade reprodutiva destes novos e promissores compostos. Dessa forma, a presente revisão tem o objetivo de abordar os principais pigmentos bacterianos, suas utilizações potenciais como drogas anticâncer, bem como os seus possíveis efeitos tóxicos, sobretudo, sobre a gônada feminina.
Palavras-chave: câncer; quimioterapia; fertilidade; compostos bioativos; bactéria
Downloads
Referências
Pope CN, Schlenk D, Baud FJ. History and basic concepts of toxicology. In: An Introduction to Interdisciplinary Toxicology. Elsevier; 2020. p. 3–15. doi: 10.1016/B978-0-12-813602-7.00001-6
Zhang T, Yan D, Yang Y, Ma A, Li L, Wang Z, et al. The comparison of animal models for premature ovarian failure established by several different source of inducers. Regul Toxicol Pharmacol. 2016;81:223–32. doi: 10.1016/j.yrtph.2016.09.002
Nieman CL, Kazer R, Brannigan RE, Zoloth LS, Chase-Landsdale PL, Kinahan K, et al. Cancer survivors and infertility: A review of a new problem and novel answers. J Support Oncol. 2006;4(4):171–8. https://www.researchgate.net/publication/7112088
Familiari G, Caggiati A, Nottola SA, Ermini M, Benedetto MR Di, Motta PM. Infertility: Ultrastructure of human ovarian primordial follicles after combination chemotherapy for hodgkin’s disease. Hum Reprod. 1993;8(12):2080–7. doi: 10.1093/oxfordjournals.humrep.a137985
Jang M, Cai L, Udeani GO, Slowing K V., Thomas CF, Beecher CWW, et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275(5297):218–20. doi: 10.1126/science.275.5297.218
Mohanty NK, Saxena S, Singh UP, Goyal NK, Arora RP. Lycopene as a chemopreventive agent in the treatment of high-grade prostate intraepithelial neoplasia. Urol Oncol Semin Orig Investig. 2005;23(6):383–5. doi: 10.1016/j.urolonc.2005.05.012
Liu X, Lin X, Zhang S, Guo C, Li J, Mi Y, et al. Lycopene ameliorates oxidative stress in the aging chicken ovary via activation of Nrf2/HO-1 pathway. Aging. 2018;10(8):2016–36. doi: 10.18632/aging.101526
Residiwati G, Azari-Dolatabad N, Tuska HSA, Sidi S, Van Damme P, Benedetti C, et al. Effect of lycopene supplementation to bovine oocytes exposed to heat shock during in vitro maturation. Theriogenology. 2021;173:48–55. doi: 10.1016/j.theriogenology.2021.07.014
Sidi S, Pascottini OB, Angel-Velez D, Azari-Dolatabad N, Pavani KC, Residiwati G, et al. Lycopene supplementation to serum-free maturation medium improves in vitro bovine embryo development and quality and modulates embryonic transcriptomic Profile. Antioxidants. 2022;344(11):3–18. doi: 10.3390/antiox11020344
Hassanpour A, Yousefian S, Askaripour M, Sharififar F, Ezzatabadipour M. Ovarian protection in cyclophosphamide-treated mice by fennel. Toxicol Reports [Internet]. 2017;4:160–4. Available from: https://doi.org/10.1016/j.toxrep.2017.03.002
Moselhy SS, Al Mslmani MAB. Chemopreventive effect of lycopene alone or with melatonin against the genesis of oxidative stress and mammary tumors induced by 7,12 dimethyl(a)benzanthracene in sprague dawely female rats. Mol Cell Biochem. 2008;319(1–2):175–80. doi: 10.1007/s11010-008-9890-6
Palomino GJQ, Sá NAR, Guerreiro DD, Gomes FDR, Silva RF, Lopes EPF, et al. Induced-damages on preantral follicles by withanolide D, a potent chemotherapy candidate are not attenuated by melatonin. Reprod Toxicol. 2021;104:125–33. doi: 10.1016/j.reprotox.2021.07.005
Fernandes SS, Coelho MS, Salas-Mellado M de las M. Bioactive compounds as ingredients of functional foods: polyphenols, carotenoids, peptides from animal and plant sources new [Internet]. Bioactive Compounds: Health Benefits and Potential Applications. Elsevier Inc.; 2018. 129–142 p. Available from: https://doi.org/10.1016/B978-0-12-814774-0.00007-4
Ran X, Zhang G, Li S, Wang J. Characterization and antitumor activity of camptothecin from endophytic fungus Fusarium solani isolated from Camptotheca acuminate. Afr Health Sci. 2017;17(2):566–74. doi: 10.4314/ahs.v17i2.34
Anwar MM, Shalaby M, Embaby AM, Saeed H, Agwa MM, Hussein A. Prodigiosin/PU-H71 as a novel potential combined therapy for triple negative breast cancer (TNBC): preclinical insights. Sci Rep [Internet]. 2020;10(1):1–15. Available from: https://doi.org/10.1038/s41598-020-71157-w
Kerr JR, Taylor GW, Rutman A, Høiby N, Cole PJ, Wilson R. Pseudomonas aeruginosa pyocyanin and 1-hydroxyphenazine inhibit fungal growth. J Clin Pathol. 1999;52(5):385–7. doi: 10.1136/jcp.52.5.385
Marrez DA, Mohamad HS. Biological activity and applications of pyocyanin produced by Pseudomonas aeruginosa. Open Access J Biomed Sci. 2020;1(4):140–4. doi: 10.38125/OAJBS.000133
Borić M, Danevčič T, Stopar D. Prodigiosin from Vibrio sp. DSM 14379; A New UV-Protective Pigment. Microb Ecol. 2011;62(3):528–36. doi: 10.1007/s00248-011-9857-0
Guryanov I, Naumenko E, Akhatova F, Lazzara G, Cavallaro G, Nigamatzyanova L, et al. Selective cytotoxic activity of prodigiosin@halloysite nanoformulation. Front Bioeng Biotechnol. 2020;8:1–13. doi: 10.3389/fbioe.2020.00424
Reis-Mansur MCPP, Cardoso-Rurr JS, Silva JVMA, Souza GR, Cardoso VS, Mansoldo FRP, et al. Carotenoids from UV-resistant Antarctic Microbacterium sp. LEMMJ01. Sci Rep. 2019;9(9554):1–14. doi: 10.1038/s41598-019-45840-6
Ram S, Mitra M, Shah F, Tirkey SR, Mishra S. Bacteria as an alternate biofactory for carotenoid production: A review of its applications, opportunities and challenges. J Funct Foods [Internet]. 2020;67:103867. Available from: https://doi.org/10.1016/j.jff.2020.103867
Malhotra V, Perry MC. Classical chemotherapy: mechanisms, toxicities and the therapeutic window. Cancer Biol Ther. 2003;2(4):1–4. https://doi.org/10.4161/cbt.199
Desai A, Qazi G, Ganju R, El-Tamer M, Singh J, Saxena A, et al. Medicinal plants and cancer chemoprevention. Curr Drug Metab. 2008;9(7):581–91. doi: 10.2174/138920008785821657
Alizadeh D, Trad M, Hanke NT, Larmonier CB, Janikashvili N, Bonnotte B, et al. Doxorubicin eliminates myeloid-derived suppressor cells and enhances the efficacy of adoptive T-cell transfer in breast cancer. Cancer Res. 2014;74(1):104–18. doi: 10.1158/0008-5472.CAN-13-1545
Borenfreund E, Babich H, Martin-alguacil A. Rapid chemosensitivity assay with human normal and tumor cells in vitro. Vitr Cell Dev Biol. 1990;26(11):1030–4. doi: 10.1007/BF02624436
Zhao R, Liu X, Yang X, Jin B, Shao C, Kang W, et al. Nanomaterial-based organelles protect normal cells against chemotherapy-induced cytotoxicity. Adv Mater. 2018;30(27):1–8. doi: 10.1002/adma.201801304
Titus S, Szymanska KJ, Musul B, Turan V, Taylan E, Garcia- Milian R, et al. Individual-oocyte transcriptomic analysis shows that genotoxic chemotherapy depletes human primordial follicle reserve in vivo by triggering proapoptotic pathways without growth activation. Sci Rep. 2021;11(407):1-10. doi: 10.1038/s41598-020-79643-x
Bellusci G, Mattiello L, Iannizzotto V, Ciccone S, Maiani E, Villani V, et al. Kinase-independent inhibition of cyclophosphamide-induced pathways protects the ovarian reserve and prolongs fertility. Cell Death Dis. 2019;10(10):1–14. doi: 10.1038/s41419-019-1961-y
Tanaka T, Utsunomiya T, Utsunomiya H, Umesaki N. Irinotecan HCl, an anticancer topoisomerase I inhibitor, frequently induces ovarian failure in premenopausal and perimenopausal women. Oncol Rep. 2008;19:1123–33. doi: 10.3892/or.19.5.1123
Tarumi W, Suzuki N, Takahashi N, Kobayashi Y, Kiguchi K, Sato K, et al. Ovarian toxicity of paclitaxel and effect on fertility in the rat. J Obstet Gynaecol Res. 2009;35(3):414–20. doi: 10.1111/j.1447-0756.2009.01023.x
Stefansdottir A, Johnston ZC, Powles-Glover N, Anderson RA, Adams IR, Spears N. Etoposide damages female germ cells in the developing ovary. BMC Cancer. 2016;16(482):1-14. doi: 10.1186/s12885-016-2505-9
Chu CS, Rubin SC. Basic Principles of chemotherapy. In: Clinical Gynecologic Oncology. Elsevier Inc.; 2018. p. 449-469.e2.
Kim S, Lee S, Park HT, Song JY, Kim T. Genomic consideration in chemotherapy-induced ovarian damage and fertility preservation. Genes. 2021;12(10):1525. doi: /10.3390/ genes12101525
Kalich-Philosoph L, Roness H, Carmely A, Fishel-Bartal M, Ligumsky H, Paglin S, et al. Cyclophosphamide triggers follicle activation and "burnout "; AS101 prevents follicle loss and preserves fertility. Sci Transl Med. 2013;5(185):1–9. doi: 10.1126/scitranslmed.3005402
Yang M, Cushman RA, Fortune JE. Anti-Mullerian hormone inhibits activation and growth of bovine ovarian follicles in vitro and is localized to growing follicles. Mol Hum Reprod. 2017;23(5):282–91. doi: 10.1093/molehr/gax010
Sonigo C, Beau I, Binart N, Grynberg M. The impact of chemotherapy on the ovaries: Molecular aspects and the prevention of ovarian damage. Int J Mol Sci. 2019;20(21):5342. doi: 10.3390/ijms20215342
Bar-Joseph H, Ben-Aharon I, Tzabari M, Tsarfaty G, Stemmer SM, Shalgi R. In vivo bioimaging as a novel strategy to detect Doxorubicin-Induced damage to gonadal blood vessels. PLoS One. 2011;6(9):1-8. doi: 10.1371/journal.pone.0023492
Meirow D, Dor J, Kaufman B, Shrim A, Rabinovici J, Schiff E, et al. Cortical fibrosis and blood-vessels damage in human ovaries exposed to chemotherapy. Potential mechanisms of ovarian injury. Hum Reprod. 2007;22(6):1626–33. doi: 10.1093/humrep/dem027
Goswami D, Conway GS. Premature ovarian failure. Human Reproduction Update. 2005;11(4):391–410. doi: 10.1093/humupd/dmi012
Ghahremani-Nasab M, Ghanbari E, Jahanbani Y, Mehdizadeh A, Yousefi M. Premature ovarian failure and tissue engineering. J Cell Physiol. 2020;235(5):4217-26. doi: 10.1002/jcp.29376
Ebrahimi M, Asbagh FA. Pathogenesis and causes of premature ovarian failure: An update. Int J Fertil Steril. 2011;5(2):54-65.
Abedal-Majed MA, Cupp AS. Livestock animals to study infertility in women. Anim Front. 2019;9(3):28–33. doi: 10.1093/af/vfz017
Bandyopadhyay S, Chakrabarti J, Banerjee S, Pal AK, Goswami SK, Chakravarty BN, et al. Galactose toxicity in the rat as a model for premature ovarian failure: An experimental approach readdressed. Hum Reprod. 2003;18(10):2031–8. doi: 10.1093/humrep/deg414.
Bromberg N, Dreyfuss JL, Regatieri C V., Palladino M V., Durán N, Nader HB, et al. Growth inhibition and pro-apoptotic activity of violacein in Ehrlich ascites tumor. Chem Biol Interact. 2010;186(1):43–52. doi: 10.1016/j.cbi.2010.04.016
Kodach LL, Bos CL, Durán N, Peppelenbosch MP, Ferreira C V., Hardwick JCH. Violacein synergistically increases 5-fluorouracil cytotoxicity, induces apoptosis and inhibits Akt-mediated signal transduction in human colorectal cancer cells. Carcinogenesis. 2006;27(3):508–16. doi: 10.1093/carcin/bgi307
El-Naggar N, El-Ewasy SM. Bioproduction, characterization, anticancer and antioxidant activities of extracellular melanin pigment produced by newly isolated microbial cell factories Streptomyces glauscescens NEAE-H. Sci Rep. 2017;7:1–19. doi: 10.1038/srep42129
Arun G, Eyini M, Gunasekaran P. Characterization and biological activities of extracellular melanin produced by Schizophyllum commune (Fries). Indian J Exp Biol. 2015;53(6):380–7.
Pavan ME, López NI, Pettinari MJ. Melanin biosynthesis in bacteria, regulation and production perspectives. Appl Microbiol Biotechnol. 2020;104(4):1357–70. doi: 10.1007/s00253-019-10245-y
Darshan N, Manonmani HK. Prodigiosin and its potential applications. J Food Sci Technol. 2015;52(9):5393–407. doi: 10.1007/s13197-015-1740-4
Azman AS, Mawang CI, Abubakar S. Bacterial pigments: The bioactivities and as an alternative for therapeutic applications. Nat Prod Commun. 2018;13(12):1747–54. doi: 10.1177/1934578X1801301240
Suryawanshi RK, Patil CD, Borase HP, Narkhede CP, Stevenson A, Hallsworth JE, et al. Towards an understanding of bacterial metabolites prodigiosin and violacein and their potential for use in commercial sunscreens. Int J Cosmet Sci. 2015;37(1):98–107. doi: 10.1111/ics.12175
Meza-Herrera CA, Vargas-Beltran F, Tena-Sempere M, González-Bulnes A, Macias-Cruz U, Veliz-Deras FG. Short-term betacarotene supplementation positively affects ovarian activity and serum insulin concentrations in a goat model. J Endocrinol Invest. 2013;36(3):185–9. doi: 10.3275/8410
Meza-Herrera CA, Reyes-Avila JM, Tena-Sempere M, Veliz-Deras FG, Macias-Cruz U, Rodriguez-Martinez R, et al. Long-term betacarotene supplementation positively affects serum triiodothyronine concentrations around puberty onset in female goats. Small Rumin Res [Internet]. 2014;116(2):176–82. Available from: http://dx.doi.org/10.1016/j.smallrumres.2013.10.017
Venil CK, Dufossé L, Renuka Devi P. Bacterial Pigments: Sustainable Compounds With Market Potential for Pharma and Food Industry. Front Sustain Food Syst. 2020;4:1–17. doi: 10.3389/fsufs.2020.00100
Courdavault V, O’Connor SE, Oudin A, Besseau S, Papon N. Towards the Microbial Production of Plant-Derived Anticancer Drugs. Trends in Cancer [Internet]. 2020;6(6):444–8. Available from: https://doi.org/10.1016/j.trecan.2020.02.004
Flores-Bustamante ZR, Rivera-0rdũa FN, Martínez-Cárdenas A, Flores-Costera LB. Microbial paclitaxel: Advances and perspectives. J Antiot. 2010;63(8):460-7. doi: 10.1038/ja.2010.83
Bilsland E, Tavella TA, Krogh R, Stokes JE, Roberts A, Ajioka J, et al. Antiplasmodial and trypanocidal activity of violacein and deoxyviolacein produced from synthetic operons. BMC Biotechnol. 2018;18(1):1-8. doi: 10.1186/s12896-018-0428-z
Aloo BN, Makumba BA, Mbega ER. The potential of Bacilli rhizobacteria for sustainable crop production and environmental sustainability. Microbiol Res [Internet]. 2019;219:1–33. Available from: https://doi.org/10.1016/j.micres.2018.10.011
Aryee AN, Agyei D, Akanbi TO. Recovery and utilization of seaweed pigments in food processing. Curr Opin Food Sci [Internet]. 2018;19:113–9. Available from: https://doi.org/10.1016/j.cofs.2018.03.013
Vila E, Hornero-Méndez D, Azziz G, Lareo C. Carotenoids from heterotrophic bacteria isolated from Fildes Peninsula, King George Island, Antarctica. Biotechnol Reports. 2018;20:1–7. doi: 10.1016/j.bre.2019.e00306
Hix LM, Lockwood SF, Bertram JS. Bioactive carotenoids: Potent antioxidants and regulators of gene expression. Redox Rep. 2004;9(4):181–91. doi: 10.1179/135100004225005967
Grune T, Lietz G, Palou A, Ross AC, Stahl W, Tang G, et al. B-Carotene is am important vitamin a source for humans. J Nutr. 2010;140:2268–85. doi: 10.3945/jn.109.119024
Lopez-Flores NM, Meza-Herrera CA, Perez-Marin C, Blache D, Arellano-Rodríguez G, Zuñiga-Garcia S, et al. Precision betacarotene supplementation enhanced ovarian function and the LH release pattern in yearling crossbred anestrous goats. Animals. 2020;10(4):1–10. doi: 10.3390/ani10040659
Yu S, Zhao Y, Feng Y, Zhang H, Li L, Shen W, et al. Β-Carotene improves oocyte development and maturation under oxidative stress in vitro. Vitr Cell Dev Biol - Anim. 2019;55(7):548–58. doi: 10.1007/s11626-019-00373-0
Taweechaipaisankul A, Jin JX, Lee S, Kim GA, Lee BC. The effects of canthaxanthin on porcine oocyte maturation and embryo development in vitro after parthenogenetic activation and somatic cell nuclear transfer. Reprod Domest Anim. 2016;51:870–6. doi: 10.1111/rda.12748
Liou GY, Storz P. Reactive oxygen species in cancer. Free Radic Res. 2010;44(5):479–96. doi: 10.3109/10715761003667554
Al-Gubory KH. Environmental pollutants and lifestyle factors induce oxidative stress and poor prenatal development. Reproductive BioMedicine Online. 2014;(29):17–31. doi: 10.1016/j.rbmo.2014.03.002
Holzapfel NP, Shokoohmand A, Wagner F, Landgraf M, Champ S, Holzapfel BM, et al. Lycopene reduces ovarian tumor growth and intraperitoneal metastatic load. Am J Cancer Res. 2017;7(6):1322–36.
Nagendraprabhu P, Sudhandiran G. Astaxanthin inhibits tumor invasion by decreasing extracellular matrix production and induces apoptosis in experimental rat colon carcinogenesis by modulating the expressions of ERK-2, NFkB and COX-2. Invest New Drugs. 2011;29(2):207–24. doi: 10.1007/s10637-009-9342-5
Takeshima M, Ono M, Higuchi T, Chen C, Hara T, Nakano S. Anti-proliferative and apoptosis-inducing activity of lycopene against three subtypes of human breast cancer cell lines. Cancer Sci. 2014;105(3):252-7. doi: 10.1111/cas.12349
Jeong Y, Lim JW, Kim H. Lycopene inhibits reactive oxugen species-mediated nf-kb signaling and induces apoptosis in pancreatic cancer cells. Nutrients. 2019;11(4):1-17. doi: doi:10.3390/nu11040762
Tran-Ly AN, Reyes C, Schwarze FWMR, Ribera J. Microbial production of melanin and its various applications. World J Microbiol Biotechnol [Internet]. 2020;36(11):1–9. Available from: https://doi.org/10.1007/s11274-020-02941-z
Singh S, Nimse SB, Mathew DE, Dhimmar A, Sahastrabudhe H, Gajjar A, et al. Microbial melanin: Recent advances in biosynthesis, extraction, characterization, and applications. Biotechnol Adv [Internet]. 2021;53(5):107773. Available from: https://doi.org/10.1016/j.biotechadv.2021.107773
Gamal Shalaby AS, Ragab TIM, Helal MMI, Esawy MA. Optimization of Bacillus licheniformis MAL tyrosinase: in vitro anticancer activity for brown and black eumelanin. Heliyon [Internet]. 2019;5(5):e01657. Available from: https://doi.org/10.1016/j.heliyon.2019.e01657
Matz C., Deines P, Boenigk J, Arndt H, Eberl L, Kjelleberg S, Ju¨rgens. Impact of violacein-producing bacteria on survival and feeding of bacterivorous nanoflagellates. Appl Environ Microbiol. 2004;70(3): 1593–9. doi: 10.1128/AEM.70.3.1593–1599.2004
Dodou HV., de Morais Batista AH, Sales GWP, de Medeiros SC, Rodrigues ML, Nogueira PCN, et al. Violacein antimicrobial activity on Staphylococcus epidermidis and synergistic effect on commercially available antibiotics. J Appl Microbiol. 2017;123(4):853–60. doi: 10.1111/jam.13547
Andrighetti-Fröhner CR, Antonio R V, Creczynski-Pasa TB, Barardi CRM, Simões CMO. Cytotoxicity and potential antiviral evaluation of violacein produced by Chromobacterium violaceum. Mem Inst Oswaldo Cruz. 2003;98(6):843–8. doi: 10.1590/s0074-02762003000600023
Ferreira CV, Bos CL, Versteeg HH, Justo GZ, Durán N, Peppelenbosch MP. Molecular mechanism of violacein-mediated human leukemia cell death. Blood. 2004;104(5):1459–64. doi: 10.1182/blood-2004-02-0594
Carvalho DD, Costa FTM, Duran N, Haun M. Cytotoxic activity of violacein in human colon cancer cells. Toxicol Vitr. 2006;20(8):1514–21. doi: 10.1016/j.tiv.2006.06.007
Masuelli L, Pantanella F, La Regina G, Benvenuto M, Fantini M, Mattera R, et al. Violacein, an indole-derived purple-colored natural pigment produced by Janthinobacterium lividum, inhibits the growth of head and neck carcinoma cell lines both in vitro and in vivo. Tumor Biol. 2016;37(3):3705–17. doi: 10.1007/s13277-015-4207-3
Mehta T, Vercruysse K, Johnson T, Ejiofor AO, Myles E, Quick QA. Violacein induces p44/42 mitogen-activated protein kinase-mediated solid tumor cell death and inhibits tumor cell migration. Mol Med Rep. 2015;12(1):1443–8. doi: 10.3892/mmr.2015.3525
Fürstner A. Chemistry and biology of roseophilin and the prodigiosin alkaloids: A survey of the last 2500 years. Angew Chem Int Ed. 2003;42(31):3582–603. doi: 10.1002/anie.200300582
Elahian F, Moghimi B, Dinmohammadi F, Ghamghami M, Hamidi M, Mirzaei SA. The anticancer agent prodigiosin is not a multidrug resistance protein substrate. DNA Cell Biol. 2013;32(3):90–7. doi: 10.1089/dna.2012.1902
Liu Y, Zhou H, Ma X, Lin C, Lu L, Liu D, et al. Prodigiosin inhibits proliferation, migration, and invasion of nasopharyngeal cancer cells. Cell Physiol Biochem. 2018;48(4):1556–62. doi: 10.1159/00049227
Silva A, Guimarães L, Ferreira E, Torres M, Silva A., Branco P, et al. Bioprospecting anticancer compounds from the marine-derived Actinobacteria Actinomadura sp. collected at the Saint Peter and Saint Paul Archipelago (Brazil). J Braz Chem Soc. 2017;28(3):465–74. doi: 10.21577/0103-5053.20160297
Gulani C, Bhattacharya S, Das A. Assessment of process parameters influencing the enhanced production of prodigiosin from Serratia marcescens and evaluation of its antimicrobial, antioxidant and dyeing potentials. Malays J Microbiol. 2012;8(2):116–22. doi: 10.21161/mjm.03612
Sudhakar C, Shobana C, Selvankumar T, Selvam K. Prodigiosin production from Serratia marcescens strain CSK and their antioxidant, antibacterial, cytotoxic effect and in silico study of caspase-3 apoptotic protein. Biotechnol Appl Biochem. 2021;1–14. doi: 10.1002/bab.2261
Wang F, Luo H, Song G, Liu C, Wang J, Xu J, et al. Prodigiosin found in Serratia marcescens y2 initiates phototoxicity in the cytomembrane. Electron J Biotechnol. 2013;16(4):1–9. doi: 10.2225/vol16-issue4-fulltext-7
Ji S, Sun R, Xu K, Man Z, Ji J, Pu Y, et al. Prodigiosin induces apoptosis and inhibits autophagy via the extracellular signal-regulated kinase pathway in K562 cells. Toxicol Vitr [Internet]. 2019;60(11):107–15. Available from: https://doi.org/10.1016/j.tiv.2019.05.003
Zhao Y, Cheng Q, Shen Z, Fan B, Xu Y, Cao Y, et al. Structure of prodigiosin from Serratia marcescens njzt-1 and its cytotoxicity on tsc2-null cells. Food Sci Technol. 2021;41:189–96. doi: 10.1590/fst.35719
Suryawanshi RK, Koujah L, Patil CD, Ames JM, Agelidis A, Yadavalli T, et al. Bacterial pigment prodigiosin demonstrates a unique antiherpesvirus activity that is mediated through inhibition of prosurvival signal transducers. J Virol. 2020;94(13):1–30. doi: 10.1128/JVI.00251-20
Suryawanshi RK, Patil CD, Koli SH, Hallsworth JE, Patil S V. Antimicrobial activity of prodigiosin is attributable to plasma-membrane damage. Nat Prod Res [Internet]. 2017;31(5):572–7. Available from: http://dx.doi.org/10.1080/14786419.2016.1195380
Krishna PS, Vani K, Prasad MR, Samatha B, Hima Bindu NSVSL, Singara Charya MA, et al. In -silico molecular docking analysis of prodigiosin and cycloprodigiosin as COX-2 inhibitors. Springerplus. 2013;2(1):1–6. http://www.springerplus.com/content/2/1/172
Montaner B, Navarro S, Piqué M, Vilaseca M, Martinell M, Giralt E, et al. Prodigiosin from the supernatant of Serratia marcescens induces apoptosis in haematopoietic cancer cell lines. Br J Pharmacol. 2000;131(3):585–93. doi: 10.1038/sj.bjp.0703614
Lapenda JCL, Alves VP, Adam ML, Rodrigues MD, Nascimento SC. Cytotoxic effect of prodigiosin, natural red pigment, isolated from Serratia marcescens UFPEDA 398. Indian J Microbiol [Internet]. 2020;60(2):182–95. Available from: https://doi.org/10.1007/s12088-020-00859-6
Lin P Bin, Shen J, Ou PY, Liu LY, Chen ZY, Chu FJ, et al. Prodigiosin isolated from Serratia marcescens in the Periplaneta americana gut and its apoptosis‑inducing activity in HeLa cells. Oncol Rep. 2019;41(6):3377–85. doi: 10.3892/or.2019.7089
Berning L, Schlütermann D, Friedrich A, Berleth N, Sun Y, Wu W, et al. Prodigiosin sensitizes sensitive and resistant urothelial carcinoma cells to cisplatin treatment. Molecules. 2021;26(5):1–17. doi: 10.3390/molecules26051294
Ashour EA, Farsi RM, Alaidaroos BA, Abdel-Moneim AME, El-Saadony MT, Osman AO, et al. Impacts of dietary supplementation of pyocyanin powder on growth performance, carcase traits, blood chemistry, meat quality and gut microbial activity of broilers. Ital J Anim Sci [Internet]. 2021;20(1):1357–72. Available from: https://doi.org/10.1080/1828051X.2021.1924087
Moayedi A, Nowroozi J, Akhavan Sepahy A. Cytotoxic effect of pyocyanin on human pancreatic cancer cell line (Panc-1). Iran J Basic Med Sci. 2018;21(8):794–9. doi: 10.22038/IJBMS.2018.27865.6799
Kumari S, Badana AK, Murali Mohan G, Shailender G, Malla RR. Reactive oxygen species: A key constituent in cancer survival. biomarker insights. 2018;13:1-9. doi: 10.1177/1177271918755391
Conklin KA. Chemotherapy-associated oxidative stress: Impact on chemotherapeutic effectiveness. Integr Cancer Ther. 2004;3(4):294–300. doi: 10.1177/1534735404270335
Colavitti R, Finkel T. Reactive oxygen species as mediators of cellular senescence. IUBMB Life. 2005;57:277–81. doi: 10.1080/15216540500091890
Laxmi M, Bhat SG. Characterization of pyocyanin with radical scavenging and antibiofilm properties isolated from Pseudomonas aeruginosa strain BTRY1. 3 Biotech. 2016;6(1):1–5. doi: 10.1007/s13205-015-0350-1
Zhao J, Wu Y, Alfred AT, Wei P, Yang S. Anticancer effects of pyocyanin on HepG2 human hepatoma cells. Lett Appl Microbiol. 2014;58:541–8. doi: 10.1111/lam.12224
Muller M. Premature cellular senescence induced by pyocyanin, a redox-active Pseudomonas aeruginosa toxin. Free Radic Biol Med. 2006;41(11):1670–7. doi: 10.1016/j.freeradbiomed.2006.09.004
Downloads
Publicado
Como Citar
Edição
Seção
Licença
Copyright (c) 2022 Ciência Animal Brasileira / Brazilian Animal Science

Este trabalho está licenciado sob uma licença Creative Commons Attribution 4.0 International License.
Autores que publicam nesta revista concordam com os seguintes termos:
- Autores mantém os direitos autorais e concedem à revista o direito de primeira publicação, com o trabalho simultaneamente licenciado sob a Licença Creative Commons Attribution que permite o compartilhamento do trabalho com reconhecimento da autoria e publicação inicial nesta revista.
- Autores têm autorização para assumir contratos adicionais separadamente, para distribuição não-exclusiva da versão do trabalho publicada nesta revista (ex.: publicar em repositório institucional ou como capítulo de livro), com reconhecimento de autoria e publicação inicial nesta revista.
- Autores têm permissão e são estimulados a publicar e distribuir seu trabalho online (ex.: em repositórios institucionais ou na sua página pessoal) a qualquer ponto antes ou durante o processo editorial, já que isso pode gerar alterações produtivas, bem como aumentar o impacto e a citação do trabalho publicado (Veja O Efeito do Acesso Livre).






























