O peixe-zebra (Danio rerio) encontra a bioética: os princípios éticos dos 10Rs na pesquisa
DOI:
https://doi.org/10.1590/1809-6891v22e-70884Resumo
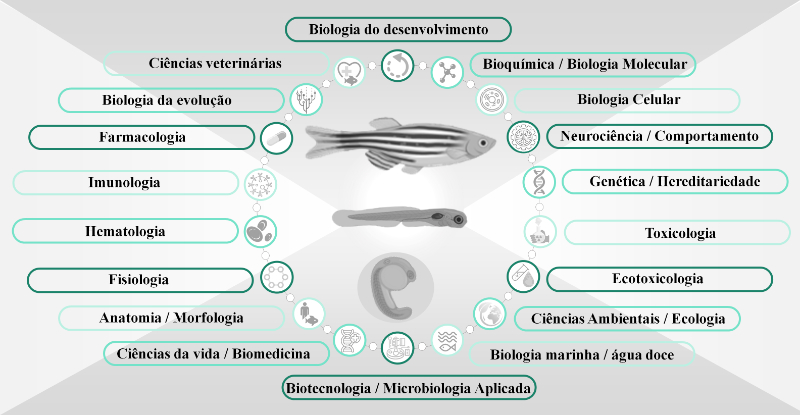
O peixe-zebra (Danio rerio) é um peixe tropical amplamente usado em pesquisas em todo o mundo. Devido ao seu uso emergente em várias áreas de pesquisa, o desenvolvimento de animais geneticamente modificados e o aumento das instalações de peixe-zebra, novos desafios éticos surgem na pesquisa com o peixe-zebra. Além disso, é necessária a conscientização da comunidade científica sobre as normas éticas e leis vigentes na pesquisa com animais. Assim, o presente estudo teve como objetivo descrever os princípios éticos de 10 Rs usando o peixe-zebra como sistema modelo em pesquisa. Os 3 Rs clássicos relativos ao bem-estar animal (substituição, redução e refinamento) e 7 Rs adicionais relacionados aos princípios científicos (registro, relatório, robustez, reprodutibilidade e relevância) e de conduta (responsabilidade e respeito) na pesquisa do peixe-zebra são apresentados e discutido criticamente. Recomendamos o uso desses 10 Rs pelos pesquisadores, instituições e Comitê de Ética Animal na regulamentação, decisão e promoção da saúde e bem-estar do peixe-zebra em pesquisas.
Palavras-chave: saúde animal; bem-estar animal; comitês de ética animal; peixe; animais de laboratório.
Downloads
Referências
Selderslaghs IW, Blust R, Witters HE. Feasibility study of the zebrafish assay as an alternative method to screen for developmental toxicity and embryotoxicity using a training set of 27 compounds. Reproductive toxicology. 2012;33(2):142-154. doi:10.1016/j.reprotox.2011.08.003
Roper C, Tanguay RL. Zebrafish as a Model for Developmental Biology and Toxicology. Handbook of Developmental Neurotoxicology (Second Edition). 2018; 143-151. doi:10.1016/B978-0-12-809405-1.00012-2
Lammer E, Carr GJ, Wendler K, Rawlings JM, Belanger SE, Braunbeck T. Is the fish embryo toxicity test (FET) with the zebrafish (Danio rerio) a potential alternative for the fish acute toxicity test?. Comparative biochemistry and physiology. Toxicology & pharmacology: CBP,2009;149(2):196-209. doi:10.1016/j.cbpc.2008.11.006
Canedo A, Rocha TL. Zebrafish (Danio rerio) using as model for genotoxicity and DNA repair assessments: historical review, current status and trends. The Science of the total environment. 2021;762:144084. doi:10.1016/j.scitotenv.2020.144084
Canedo A, de Jesus LWO, Bailão EFLC, Rocha TL. Micronucleus test and Nuclear Abnormality assay in zebrafish (Danio rerio): past, present and future trends. Environment Pollution. 2021:290:118019. doi:10.1016/j.envpol.2021.118019
Cristiano W, Lacchetti I, Mancini L, Corti M, Di Domenico K, Di Paolo C, Hollert H, Carere M. Promoting zebrafish embryo tool to identify the effects of chemicals in the context of Water Framework Directive monitoring and assessment. Microchemical Journal. 2019:149:104035. doi:10.1016/j.microc.2019.104035
Ribeiro RX, da Silva Brito R, Pereira AC, Monteiro KBES, Gonçalves BB, Rocha TL. Ecotoxicological assessment of effluents from Brazilian wastewater treatment plants using zebrafish embryotoxicity test: A multi-biomarker approach. The Science of the total environment. 2020;735:139036. doi:10.1016/j.scitotenv.2020.139036
Nowik N, Podlasz P, Jakimiuk A, Kasica N, Sienkiewicz W, Kaleczyc J. Zebrafish: an animal model for research in veterinary medicine. Polish journal of veterinary sciences. 2015;18(3):663-674. doi:10.1515/pjvs-2015-0086
Parichy DM. The Natural History of Model Organisms: Advancing biology through a deeper understanding of zebrafish ecology and evolution. eLife 2015;4:e05635. doi: 10.7554/eLife.05635.
Sieber S, Grossen P, Bussmann J, et al. Zebrafish as a preclinical in vivo screening model for nanomedicines. Advanced drug delivery reviews. 2019;151-152:152-168. doi:10.1016/j.addr.2019.01.001
Pereira AC, Gomes T, Ferreira Machado MR, Rocha TL. The zebrafish embryotoxicity test (ZET) for nanotoxicity assessment: from morphological to molecular approach. Environmental pollution. 2019;252(Pt B):1841-1853. doi:10.1016/j.envpol.2019.06.100
Dooley K, Zon LI. Zebrafish: a model system for the study of human disease. Current opinion in genetics & development. 2000;10(3):252-256. doi:10.1016/s0959-437x(00)00074-5
Bailone RL, R, Fukushima HCS, Fernandes BHV, De Aguiar LK, Corrêa T, Janke H, Setti PG, Roça RO, Borra RC. Zebrafish as an alternative animal model in human and animal vaccination research. Laboratory Animal Research. 2020;36(13):1-10. doi:10.1186/s42826-020-00042-4
Bailone RL, Aguiar LKD, Roca RDO, Borra RC, Corrêa T, Janke H, Fukushima HCS. Zebrafish as an animal model for food safety research: trends in the animal research. Food Biotechnology. 2019;33(4): 283-302. http://dx.doi.org/10.1080/08905436.2019.1673173
Baxendale S, van Eeden F, Wilkinson R. “The Power of Zebrafish in Personalised Medicine.” Advances in experimental medicine and biology. 2017;1007:179-197. doi:10.1007/978-3-319-60733-7_10
Trigueiro, N., Canedo, A., Braga, D., Luchiari, A. C., Rocha, T. L., 2020. Zebrafish as an Emerging Model System in the Global South: Two Decades of Research in Brazil. Zebrafish. 10.1089/zeb.2020.1930. Advance online publication. doi:10.1089/zeb.2020.1930
Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Developmental dynamics: an official publication of the American Association of Anatomists. 1995;203(3):253-310. doi:10.1002/aja.1002030302
Aksoy YA, Nguyen DT, Chow S, et al. Chemical reprogramming enhances homology-directed genome editing in zebrafish embryos. Communications biology. 2019;2:198. doi:10.1038/s42003-019-0444-0
Fernandes MR, Pedroso AR. Animal experimentation: A look into ethics, welfare and alternative methods. Revista da Associação Medica Brasileira (1992). 2017;63(11):923-928. doi:10.1590/1806-9282.63.11.923
Cornet C, Calzolari S, Miñana-Prieto R, et al. ZeGlobalTox: An Innovative Approach to Address Organ Drug Toxicity Using Zebrafish. International journal of molecular sciences. 2017;18(4):864. doi:10.3390/ijms18040864
OECD, 2013. OECD. OECD guidelines for the testing of chemicals. Section 2: Effects on Biotic Systems Test No. 236: Applicability of the Fish Embryo Acute Toxicity (FET) Test. Organization for Economic Cooperation and Development, Paris, France.
Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biology. 2010;8(6):e1000412. doi:10.1371/journal.pbio.1000412
Council of Europe, 1986, European convention for the protection of vertebrate animals used for experimental and other scientific purposes, European Treaty Series 123, Council of Europe, Strasbourg.
European Union, 2010, ‘Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes’, Official Journal of the European Union 276, 33–79.
Basel Declaration Society, 2010, Basel Declaration: A call for more trust, transparency and communication on animal research, Basel Declaration Committee, Basel.
National Research Council, 2011, Guide for the care and use of laboratory animals: Eighth edition, National Academic Press, Washington, DC.
Society of Environmental Toxicology and Chemistry, n.d., SETAC code of ethics, viewed 30 August 2021, from https://www.setac.org/?page=SETACEthics
American Fisheries Society, 2014, ‘Guidelines for the use of fishes in research’, American Fisheries Society, Bethesda, MD.
Sloman KA, Bouyoucos IA, Brooks EJ, Sneddon LU. Ethical considerations in fish research. Journal of Fish Biology. 2019;94(4):556-577. https://doi.org/10.1111/jfb.13946
Canadian Council on Animal Care, 2005, Guidelines on: The care and use of fish in research, teaching and testing, Canadian Council on Animal Care, Ottawa.
Osborne N, Paull G, Grierson A, et al. Report of a Meeting on Contemporary Topics in Zebrafish Husbandry and Care. Zebrafish. 2016;13(6):584-589. doi:10.1089/zeb.2016.1324
Lidster K, Readman GD, Prescott MJ, Owen SF. International survey on the use and welfare of zebrafish Danio rerio in research. Journal of Fish Biology. 2017;90(5):1891-1905. https://doi.org/10.1111/jfb.13278
Días L, Zambrano E, Flores ME, Contreras M, Crispin JC, Alemán G, Bravo C, Armenta A, Valdés VJ, Tovar A, gamba G, Barrios-Payán J, Bobadilla NA. Ethical considerations in animal research: The principle of 3R's. Revista de investigacion clinica; organo del Hospital de Enfermedades de la Nutricion. 2021;73(4):199-209. doi:10.24875/ric.20000380
Ford KA. Refinement, Reduction, and Replacement of Animal Toxicity Tests by Computational Methods. ILAR J. 2016;57(2):226-233. doi:10.1093/ilar/ilw031
Schaeck M, Van den Broeck W, Hermans K, Decostere A. Fish as research tools: alternatives to in vivo experiments. Alternatives to laboratory animals: ATLA. 2013;41(3):219-229. doi:10.1177/026119291304100305
USEPA, 2020. New approach methods work plan: Reducing use of animals in chemical testing. U.S. Environmental Protection Agency, Washington, DC. EPA 615B2001. https://www.epa.gov/sites/default/files/2020-06/documents/epa_nam_work_plan.pdf
OECD, 2010. OECD Guidelines for the Testing of Chemicals. Section 2: Effects on Biotic Systems Test No. 233: Sediment-water Chironomid life-cycle toxicity test using spiked water or spiked sediment. Organization for Economic Cooperation and Development, Paris, France.
OECD, 2012. OECD Guidelines for the Testing of Chemicals. Section 2: Effects on Biotic Systems Test No. 211: Daphnia magna Reproduction Test. Organization for Economic Cooperation and Development, Paris, France.
OECD, 2016a. OECD Guidelines for the Testing of Chemicals. Section 2: Effects on Biotic Systems Test No. 222: Earthworm Reproduction Test (Eisenia fetida/Eisenia andrei). Organization for Economic Cooperation and Development, Paris, France.
OECD, 2016b. OECD Guidelines for the Testing of Chemicals. Section 2: Effects on Biotic Systems Test No. 243: Lymnaea stagnalis Reproduction Test. Organization for Economic Cooperation and Development, Paris, France.
OECD, 2019. OECD Guidelines for the Testing of Chemicals. Section 2: Effects on Biotic Systems Test No. 203: Fish, acute toxicity testing. Organization for Economic Cooperation and Development, Paris, France.
Streisinger G, Walker C, Dower N, Knauber D, Singer F. Production of clones of homozygous diploid zebra fish (Brachydanio rerio). Nature. 1981;291(5813):293-296. doi:10.1038/291293a0
Eisen JS. History of zebrafish research. The Zebrafish in Biomedical Research Biology Husbandry, Diseases and Research Applications. 2020: 3-14. doi: 10.1016/B978-0-12-812431-4.00001-4
Khan FR, Alhewairini SS. Zebrafish (Danio rerio) as a model organismo. In: Streba L, Gheonea DI, Schenker M. Current Trends in cancer mangement. IntechOpen. 2018. doi: 10.5772/intechopen.81517
Howe K, Clark MD, Torroja CF, Torrance J, Berthelot C, Muffato M, Collins JE, Humphray S, Mclaren K, Mathews L, et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature. 2013; 496:498-503. https://doi.org/10.1038/nature12111
ZFIN, 2021. Zebrafish strains. University of Oregon. EPA 615B2001. https://zfin.org/zf_info/zfbook/zfstrn.html. Access in 14 sep 2021.
Lee KY, Jang GH, Byun CH, Jeun M, Searson PC, Lee KH. Zebrafish models for functional and toxicological screening of nanoscale drug delivery systems: promoting preclinical applications. Bioscience reports. 2017;37(3):BSR20170199. doi:10.1042/BSR20170199
Belanger SE, Rawlings JM, Carr GJ. Use of fish embryo toxicity tests for the prediction of acute fish toxicity to chemicals. Environmental toxicology and chemistry. 2013;201332, 1768-1783. doi:10.1002/etc.2244
Braunbeck T, Kais B, Lammer E, Otte J, Schneider K, Stengel D, Strecker R. The fish embryo test (FET): origin, applications, and future. Environmental science and pollution research international. 2014; 22, 16247-16261. doi:10.1007/s11356-014-3814-7
Sneddon LU, Halsey LG, Bury NR. Considering aspects of the 3Rs principles within experimental animal biology. The Journal of experimental biology. 2017;220(Pt 17):3007-3016. doi:10.1242/jeb.147058
Bayne K, Ramachandra GS, Rivera EA, Wang J. The Evolution of Animal Welfare and the 3Rs in Brazil, China, and India. Journal of the American Association for Laboratory Animal Science. 2015;54(2):181-191.
Rácz A, Allan B, Dwyer T, Thambithurai D, Crespel A, Killen SS. Identification of Individual Zebrafish (Danio rerio): A Refined Protocol for VIE Tagging Whilst Considering Animal Welfare and the Principles of the 3Rs. Animals. 2021;11(3):616. https://doi.org/10.3390/ani11030616
Workman P, Aboagye E, Balkwill F. et al. Guidelines for the welfare and use of animals in cancer research. British Journal of Cancer, 2010;102(11):1555–1577. doi:10.1038/sj.bjc.6605642
Rinkwitz S, Mourrain P, Becker TS. Zebrafish: An integrative system for neurogenomics and neurosciences. Progress in Neurobiology, 2011;93(2):231-243. doi:10.1016/j.pneurobio.2010.11.003
Garcia GR, Noyes PD, Tanguay RL. Advancements in zebrafish applications for 21st century toxicology. Pharmacology and Therapeutics, 2016;161:11-21. doi: 10.1016/j.pharmthera.2016.03.009.
Seixas MM. et al. Consciência Na Substituição Do Uso De Animais No Ensino: Aspectos Históricos, Éticos E De Legislação. Revista Brasileira de Direito Animal, 2014; 5(6):71-96. doi:10.9771/rbda.v5i6.11073
Augustsson, H, van de Weerd, HA, Kruitwagen, CL, Baumans, V. Effect of enrichment on variation and results in the light/dark test. Laboratory Animals, 2003;7(4), 328-340. doi:10.1258/002367703322389898
Russell WMS, Burch RL. The Principles of Humane Experimental Technique. special ed ed. [s.l: s.n.].
Huntingford FA, Adams C, Braithwaite VA, Kadri S, Pottinger TG, Sandoe P, Turnbull JF. Current issues in fish welfare. Journal of Fish Biology, 2006;68(2), 332-372. doi:10.1111/j.0022-1112.2006.001046.x
Stevens CH, Reed BT, Hawkins P. Enrichment for Laboratory Zebrafish-A Review of the Evidence and the Challenges. Animals (Basel). 2021;11(3):698. doi:10.3390/ani11030698
Hawkins P, Dennison N, Goodman G, et al. Guidance on the severity classification of scientific procedures involving fish: Report of a Working Group appointed by the Norwegian Consensus-Platform for the Replacement, Reduction and Refinement of animal experiments (Norecopa). Laboratory Animals, 2011;45(4):219-224. doi:10.1258/la.2011.010181
Collymore C, Tolwani RJ, Rasmussen S. The behavioral effects of single housing and environmental enrichment on adult zebrafish (Danio rerio). Journal of the American Association for Laboratory Animal Science, 2015;54(3):280-285.
Kistler C, Hegglin D, Wurbel H, Konig B, Preference for structured environment in zebrafish (Danio rerio) and checker barbs (Puntius oligolepis). Applied Animal Behaviour Science, 2011, 135(4);318–327. doi: 10.1016/j.applanim.2011.10.014
White LJ, Thomson JS, Pounder KC, Coleman RC, Sneddon LU. The impact of social context on behaviour and the recovery from welfare challenges in zebrafish, Danio rerio. Animal Behaviour, 2017;132:189-199. doi:10.1016/j.anbehav.2017.08.017
Engeszer RE, Patterson LB, Rao AA, Parichy DM. Zebrafish in the wild: A review of natural history and new notes from the field. Zebrafish, 2007;4(1):21-40. doi: 10.1089/zeb.2006.9997.
Best J, Adatto I, Cockington J, James A, Lawrence C. A novel method for rearing first-feeding larval zebrafish: Polyculture with type L saltwater rotifers (Brachionus plicatilis). Zebrafish, 2010;7(3):289-295. doi:10.1089/zeb.2010.0667
CONSTITUIÇÃO FEDERAL. LEI No 11.794 DE 8 DE OUTUBRO DE 2008.Brasil, 2008. Disponível em: http://www.planalto.gov.br/ccivil_03/_ato2007-2010/2008/lei/l11794.htm
Neiffer DL, Stamper MA. Fish sedation, anesthesia, analgesia, and euthanasia: Considerations, methods, and types of drugs. ILAR Journal, 2009;50(4):343-360. doi:10.1093/ilar.50.4.343
Dammski AP, Muller BR, Gaya C, Regonato D. Zebrafish: Manual de Criação em Biotério. Curitiba: Universidade Federal do Paraná. 2011;1-107.
Trevarrow B, Robison B. Genetic backgrounds, standard lines, and husbandry of zebrafish. Methods in cell biology. 2004;77:599-616. doi:10.1016/s0091-679x(04)77032-6
Martins S, Monteiro JF, Vito M, Weintraub D, Almeida J, Certal AC. Toward an Integrated Zebrafish Health Management Program Supporting Cancer and Neuroscience Research. Zebrafish, 2016a; 13(00):S47–S55. doi: 10.1089/zeb.2015.1198
Martins T, Valentim AM, Pereira N, Antunes LM. Anaesthesia and analgesia in laboratory adult zebrafish: A question of refinement. Laboratory Animals, 2016b; 50(6), 476-488. doi:10.1177/0023677216670686
Readman GD, Owen SF, Murrell JC, Knowles TG. Do fish perceive anaesthetics as aversive?. PLoS One. 2013;8(9):e73773. doi:10.1371/journal.pone.0073773
Valentim AM, Félix LM, Carvalho L, Diniz E, Antunes LM. A new anaesthetic protocol for adult zebrafish (Danio rerio): Propofol combined with lidocaine. PLoS ONE, 2016;11(1):e0147747. doi:10.1371/journal.pone.0147747
Martins T, Valentim A, Pereira N, Antunes LM. Anaesthetics and analgesics used in adult fish for research: A review. Laboratory Animals, 2019; 53(4): 325-341. doi: 10.1177 / 0023677218815199
Xing L, Quist TS, Stevenson TJ, Dahlem TJ, Bonkowsky JL. Rapid and efficient zebrafish genotyping using PCR with high-resolution melt analysis. Journal of Visualized Experiments, 2014;(84):e51138. doi:10.3791/51138
Neiffer DL, Stamper MA. Fish sedation, analgesia, anesthesia, and euthanasia: considerations, methods, and types of drugs. ILAR journal. 2009;50(4):343-360. doi:10.1093/ilar.50.4.343
Schroeder PG, Sneddon LU. Exploring the efficacy of immersion analgesics in zebrafish using an integrative approach. Applied Animal Behaviour Science, 2017;187:93-102. doi:10.1016/j.applanim.2016.12.003
De Lombaert MC, Rick EL, Krugner-Higby LA, Wolman MA. Behavioral characteristics of adult zebrafish (Danio rerio) after MS222 anesthesia for fin excision. Journal of the American Association for Laboratory Animal Science, 2017;56(4):377-381.
Le Vin AL, Adam A, Tedder A, Arnold KE, Mable BK. Validation of swabs as a non-destructive and relatively non-invasive DNA sampling method in fish. Molecular Ecology Resources, 2011;11(1):107-9. doi: 10.1111 / j.1755-0998.2010.02909.x.
Breacker C, Barber I, Norton WH, McDearmid JR, Tilley CA. A Low-Cost Method of Skin Swabbing for the Collection of DNA Samples from Small Laboratory Fish. Zebrafish. 2017;14(1):35-41. doi: 10.1089/zeb.2016.1348.
Campanella JJ, Smalley JV. A minimally invasive method of piscine tissue collection and an analysis of long-term field-storage conditions for samples. BMC Genetics, 2006,7, 5-8. doi:10.1186/1471-2156-7-32
Tilley, C.A., Carreño Gutierrez, H., Sebire, M. et al. Skin swabbing is a refined technique to collect DNA from model fish species. Scientific Reports. 2020;10(1):1-17. doi:10.1038/s41598-020-75304-1
Mason, G. Stereotypic behaviour in captive animals: Fundamentals and implications for welfare and beyond. Stereotypic Animal Behaviour: Fundamentals and Applications to Welfare: Second Edition, 2006:326-356.
Gouveia K, Hurt JL. Optimising reliability of mouse performance in behavioural testing: The major role of non-aversive handling. Scientific Reports, 2017;7:1-12. doi.org/10.1038/srep44999
Mulder A. Journal of Applied Animal Welfare Effects of Environmental Enrichment for Mice: Variation in Experimental Results. Journal of Applied Animal Welfare Science, 2010;8705(776099595):7-41.
Strech D, Dirnagl U. 3Rs missing: animal research without scientific value is unethical. BMJ Open Science. 2019; 3(1)e000048. doi:10.1136/BMJOS-2018-000048
Percie du Sert, N., Hurst, V., Ahluwalia, A., Alam, S., Avey, M. T., Baker, M., Browne, W. J., Clark, A., Cuthill, I. C., Dirnagl, U., Emerson, M., Garner, P., Holgate, S. T., Howells, D. W., Karp, N. A., Lazic, S. E., Lidster, K., MacCallum, C. J., Macleod, M., Pearl, E. J., … Würbel, H. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS biology. 2020;18(7), e3000410. https://doi.org/10.1371/journal.pbio.3000410
Wieschowski S, Silva DS, Strech D. Animal Study Registries: Results from a Stakeholder Analysis on Potential Strengths, Weaknesses, Facilitators, and Barriers. PLoS biology. 2016;14(11):1-12. doi:10.1371/journal.pbio.2000391
Séguret A, Collignon B, Halloy J. Strain differences in the collective behaviour of zebrafish (Danio rerio) in heterogeneous environment. Royal Society open science. 2016;3(10):160451. doi:10.1098/rsos.160451
Lobban, C. S and Schefter, M., 2021. Write Scientific Reports – The Library : University of Waikato [WWW Document]. Cambridge Univ. Press Cambridge. URL https://www.waikato.ac.nz/library/guidance/guides/write-scientific-reports#Elementsofa ScientificReport (accessed 9.17.21).
Mogil J, Macleod M. No publication without confirmation. Nature. 2017; 542:409-411. doi:10.1038/542409a.
Needleman I, Moher D, Altman DG, Schulz KF, Moles DR, Worthington H. Improving the clarity and transparency of reporting health research: a shared obligation and responsibility. Journal of dental research. 2008;87(10):894-895. doi:10.1177/154405910808701013
De Abreu MS, Giacomini ACVV, Echevarria DJ, Kalueff AV. Legal aspects of zebrafish neuropharmacology and neurotoxicology research. Regulatory Toxicology and Pharmacology. 2018;101: 65-70. doi:10.1016/j.yrtph.2018.11.007
Steward O. A Rhumba of "R's": Replication, Reproducibility, Rigor, Robustness: What Does a Failure to Replicate Mean?. eNeuro. 2016;3(4):ENEURO.0072-16.2016. doi:10.1523/ENEURO.0072-16.2016
Resnik DB, Shamoo AE. Reproducibility and Research Integrity. Account Res. 2017;24(2):116-123. doi:10.1080/08989621.2016.1257387
Ioannidis JP. How to make more published research true. PLoS Medicine. 2014;11(10):e1001747. doi:10.1371/journal.pmed.1001747
Freedman LP, Cockburn IM, Simcoe TS. The Economics of Reproducibility in Preclinical Research. PLoS Biol. 2015;13(6):e1002165. doi:10.1371/journal.pbio.1002165
Gerlai R. Reproducibility and replicability in zebrafish behavioral neuroscience research. Pharmacology, biochemistry, and behavior. 2019;178:30-38. doi:10.1016/j.pbb.2018.02.005
OECD, 1992. OECD. OECD guidelines for the testing of chemicals. Section 2: Effects on Biotic Systems Test No. 203: Fish, Acute Toxicity Test. Organization for Economic Cooperation and Development, Paris, France.
OECD, 2000. OECD. OECD guidelines for the testing of chemicals. Section 2: Effects on Biotic Systems Test No. 215: Fish, Juvenile Growth test. Organization for Economic Cooperation and Development, Paris, France.
OECD, 2009. OECD. OECD guidelines for the testing of chemicals. Section 2: Effects on Biotic Systems Test No. 230: 21-day Fish Assay. Organization for Economic Cooperation and Development, Paris, France.
OECD, 2018. OECD. OECD guidelines for the testing of chemicals. Section 2: Effects on Biotic Systems Test No. 234: Fish Sexual Development Test (FSDT). Organization for Economic Cooperation and Development, Paris, France.
Andersen ML, Winter LMF. Animal models in biological and biomedical research - experimental and ethical concerns. Anais da Academia Brasileira de Ciencias. 2019;91(suppl 1):e20170238. doi:10.1590/0001-3765201720170238
Klein J. Improving the reproducibility of findings by updating research methodology Quality & quantity. 2021;1-13. doi:10.1007/s11135-021-01196-6
Mehić B Professor. Bioethical Principles of Biomedical Research Involving Animals. Bosnian Journal of Basic Medical Sciences. 2011;11(3):145-146.
Veldman M, Lin S. Zebrafish as a Developmental Model Organism for Pediatric Research. Pediatric research. 2008; 64:470-476 doi:10.1203/PDR.0b013e318186e609
Holtzman NG, Iovine MK, Liang JO, Morris J. Learning to Fish with Genetics: A Primer on the Vertebrate Model Danio rerio. Genetics. 2016;203(3):1069-1089. doi:10.1534/genetics.116.190843
Basnet RM, Zizioli D, Taweedet S, Finazzi D, Memo M. Zebrafish Larvae as a Behavioral Model in Neuropharmacology. Biomedicines. 2019;7(1):23. doi:10.3390/biomedicines7010023
Kari G, Rodeck U, Dicker AP. Zebrafish: an emerging model system for human disease and drug discovery. Clinical pharmacology and therapeutics.2007;82(1):70-80. doi:10.1038/sj.clpt.6100223
Gut P, Reischauer S, Stainier DYR, Arnaout R. Little fish, big data: zebrafish as a model for cardiovascular and metabolic disease. Physiological reviews. 2017;97(3):889-938. doi:10.1152/physrev.00038.2016
Giannaccini M, Cuschieri A, Dente L, Raffa V. Non-mammalian vertebrate embryos as models in nanomedicine. Nanomedicine: nanotechnology, biology, and medicine. 2014;10(4):703-719. doi:10.1016/j.nano.2013.09.010
Dai YJ, Jia YF, Chen N, et al. Zebrafish as a model system to study toxicology. Environmental toxicology and chemistry. 2014;33(1):11-17. doi:10.1002/etc.2406
Cui C, Benard EL, Kanwal Z, et al. Infectious disease modeling and innate immune function in zebrafish embryos. Methods in cell biology. 2011;105:273-308. doi:10.1016/B978-0-12-381320-6.00012-6
Lam SH, Chua HL, Gong Z, Lam TJ, Sin YM. Development and maturation of the immune system in zebrafish, Danio rerio: a gene expression profiling, in situ hybridization and immunological study. Developmental and comparative immunology. 2004;28(1):9-28. doi:10.1016/s0145-305x(03)00103-4
National Centre for the Replacement Refinement and Reduction of Animals in Research, NC3Rs. Responsibility in the use of animals in bioscience research: expectations of the major research councils and charitable funding bodies [Internet]. 2019. Available from: https://nc3rs.org.uk/sites/default/files/documents/Guidelines/Responsibility in the use of animals in bioscience research 2019.pdf
National Health and Medical Research Council, NHMRC; Australian Research Council. Australian code for the care and use of animals for scientific purposes. Vol. 2013. 2021. 86 p.
BRASIL. Resolução Normativa CONCEA n. 51, de 19 de maio de 2021. Dispõe sobre a instalação e o funcionamento das Comissões de Ética no Uso de Animais - CEUAs e dos biotérios ou instalações animais. https://www.in.gov.br/web/dou/-/resolucao-normativa-concea-n-51-de-19-de-maio-de-2021-321534226
BRASIL. (a) Resolução Normativa CONCEA n. 30, de 02.02.2016. Baixa a Diretriz Brasileira para o Cuidado e a Utilização de Animais em Atividades de Ensino ou de Pesquisa Científica – DBCA.
BRASIL. (b) Resolução Normativa CONCEA n. 32, de 06.09.2016. Baixa as Diretrizes de Integridade e de Boas Práticas para Produção, Manutenção ou Utilização de Animais em Atividades de Ensino ou Pesquisa Científica.
Ogden BD. Principles of animal research: replacement, reduction, refinement, and responsibility. Anim Law [Internet]. 1996;2:167–70. Available from: https://heinonline.org/HOL/Page?public=%0Atrue&handle=hein.journals/anim2&div=12&start_page=167&collection=%0Ajournals&set_as_cursor=0&men_tab=srchresults
Franco AL, Nogueira MNM, Sousa NGK, da Frota MF, Fernandes CMS, Serra M da C. Pesquisas em animais: uma reflexão bioética. Acta Bioethica. 2014;20(2):247-53. doi:10.4067/S1726-569X2014000200012.
McLeod C, Hartley S. Responsibility and Laboratory Animal Research Governance. Science Technology Human Values. 2018;43(4):723-41. doi:10.1177/0162243917727866
Reed B; Jennings M. Guidance on the housing and care of Zebrafish (Danio rerio). Available from: https://www.aaalac.org/pub/?id=E9019693-90EC-FC4A-526E-E8236CC13B28
Zagorac I. Fritz Jahr’s Bioethical Imperative. Synth Philos. 2011;51:141-50.
MacRae CA, Peterson RT. Zebrafish as tools for drug discovery. Nature reviews. Drug discovery. 2015;14(10):721-731. doi:10.1038/nrd4627
Message R, Greenhough B. "But It's Just a Fish": Understanding the Challenges of Applying the 3Rs in Laboratory Aquariums in the UK. Animals (Basel). 2019;9(12):1075. Published 2019 Dec 3. doi:10.3390/ani9121075
Bambino K, Chu J. Zebrafish in Toxicology and Environmental Health. Current topics in developmental biology. 2017;124:331-367. doi:10.1016/bs.ctdb.2016.10.007
Bertoncello KT, Bonan CD. Zebrafish as a tool for the discovery of anticonvulsant compounds from botanical constituents. European journal of pharmacology. 2021;908:174342. doi:10.1016/j.ejphar.2021.174342
Vierstraete J, Fieuws C, Willaert A, Vral A, Claes KBM. Zebrafish as an in vivo screening tool to establish PARP inhibitor efficacy. DNA Repair. 2021:97:103023. doi:10.1016/j.dnarep.2020.103023
Downloads
Publicado
Como Citar
Edição
Seção
Licença
Copyright (c) 2022 Ciência Animal Brasileira

Este trabalho está licenciado sob uma licença Creative Commons Attribution 4.0 International License.
Autores que publicam nesta revista concordam com os seguintes termos:
- Autores mantém os direitos autorais e concedem à revista o direito de primeira publicação, com o trabalho simultaneamente licenciado sob a Licença Creative Commons Attribution que permite o compartilhamento do trabalho com reconhecimento da autoria e publicação inicial nesta revista.
- Autores têm autorização para assumir contratos adicionais separadamente, para distribuição não-exclusiva da versão do trabalho publicada nesta revista (ex.: publicar em repositório institucional ou como capítulo de livro), com reconhecimento de autoria e publicação inicial nesta revista.
- Autores têm permissão e são estimulados a publicar e distribuir seu trabalho online (ex.: em repositórios institucionais ou na sua página pessoal) a qualquer ponto antes ou durante o processo editorial, já que isso pode gerar alterações produtivas, bem como aumentar o impacto e a citação do trabalho publicado (Veja O Efeito do Acesso Livre).






























