Abstract
Bovine semen cryopreservation is a fundamental procedure in animal reproduction. However, it is associated with the production of reactive oxygen species (ROS), which can cause significant damage to spermatozoa, leading to infertility and reduced sperm quality. In this context, using seminal diluents enriched with antioxidants presents a promising strategy for minimizing or even reversing these harmful effects. This study aimed to evaluate the effects of different concentrations of pequi (Caryocar coriaceum) esters as a supplement to bovine semen cryopreservation diluents. Forty-two ejaculates were collected from six bulls that had undergone an andrological examination and were deemed suitable. The samples were analyzed, diluted in Tris-egg yolk medium, supplemented with pequi esters (0.0, 0.5, 1.0, and 1.5 mL/L), packaged, and cryopreserved. The sperm parameters evaluated in the post-thaw semen included the thermoresistance, plasma membrane functionality, cleavage and blastocyst rates, and quantification of reduced glutathione and malondialdehyde. The results showed that the concentrations of 1.0 and 1.5 mL/L of pequi esters significantly reduced malondialdehyde levels in cryopreserved semen. However, no significant effects of pequi esters were observed on motility parameters, sperm functionality, cleavage and blastocyst rates, or on the quantification of reduced glutathione. Supplementation with pequi ester (1.0 and 1.5 mL/L) in the cryopreservation of bovine semen may be beneficial in minimizing lipid peroxidation effects, as evidenced by the reduction in malondialdehyde concentration after thawing.
Keywords:
Curraleiro Pé Duro; reactive oxygen species; malondialdehyde
Resumo
A criopreservação de sêmen bovino é um procedimento fundamental na reprodução animal, mas está associada à produção de espécies reativas de oxigênio (ROS), que podem causar danos significativos aos espermatozoides. Esses danos estão relacionados à infertilidade e à diminuição da qualidade espermática. Nesse contexto, a utilização de diluidores seminais enriquecidos com antioxidantes representa uma estratégia promissora para minimizar ou até reverter esses efeitos deletérios. O objetivo desse estudo foi avaliar os efeitos de diferentes concentrações de ésteres de pequi (Caryocar coriaceum) como suplementação ao diluente de criopreservação de sêmen bovino. Foram coletados 42 ejaculados de seis touros, submetidos ao exame andrológico e considerados aptos. As amostras foram analisadas, diluídas em Tris-gema, suplementadas com ésteres de pequi (0; 0,5; 1,0 e 1,5 mL/L), envasadas e criopreservadas. Os parâmetros espermáticos avaliados no sêmen pós-descongelamento incluíram o teste de termorresistência fisiológica (TTR), a funcionalidade da membrana plasmática (HOST), as taxas de clivagem e blastocisto, bem como a quantificação de glutationa reduzida e de malondialdeído (MDA). Os resultados mostraram que as concentrações de 1,0 e 1,5 mL/L de ésteres de pequi reduziram significativamente a concentração de malondialdeído no sêmen criopreservado. No entanto, não foram observados efeitos significativos dos ésteres de pequi nos parâmetros de motilidade, funcionalidade espermática, taxas de clivagem e blastocisto, ou na quantificação da glutationa reduzida. Conclui-se que a suplementação com os ésteres de pequi (1,0 e 1,5 mL/L) na criopreservação de sêmen bovino pode ser benéfica para minimizar os efeitos da peroxidação lipídica dos espermatozoides, uma vez que houve redução na concentração de malondialdeído pós-descongelamento.
1. Introduction
Cryopreservation of bovine semen is a crucial process for assisted reproduction, but it generates significant oxidative stress, leading to an imbalance between the production of oxidative species and cellular antioxidative defenses. This stress can compromise sperm physiology, resulting in infertility or subfertility in bulls(1). Free radicals, which are present under normal physiological conditions, play essential roles in various stages of reproduction, including spermatogenesis and fertilization(2). Therefore, to mitigate the damage caused by cryopreservation, the supplementation of antioxidants in seminal diluents is a promising approach to preserve semen quality and maintain the reproductive capacity of spermatozoa(1,3,4).
In this context, pequi oil (Caryocar coriaceum) has emerged as a potential antioxidant due to its high content of unsaturated fatty acids and compounds such as gallic acid, which are known for their ability to eliminate reactive oxygen species (ROS)(5,6). Previous studies have shown that pequi oil is effective in reducing oxidative damage in cells and in protecting against lipid peroxidation and DNA damage(7). Given these properties, this study aims to evaluate pequi esters as antioxidants in the cryopreservation of bovine semen, assessing their impact on critical sperm quality parameters such as thermoresistance via the thermoresistance test (TTR), plasma membrane functionality via the hypo-osmotic swelling test (HOST), cleavage rates, and blastocyst formation as well as the quantification of reduced glutathione (GSH) and malondialdehyde (MDA).
By exploring the use of pequi esters in sperm cryopreservation, this study not only addresses a gap in the scientific literature but also proposes a viable alternative for enhancing reproductive efficiency in bulls, highlighting the integration of reproductive biotechnology with the use of natural antioxidants.
2. Material and methods
All procedures performed on the animals complied with European legislation on animal experimentation (Directive 2010/63/EU) and Brazilian legislation on animal research (Law 11.794, October 8, 2008). The protocol described in this study was approved by the Ethics Committee for the Use of Animals (CEUA) of EMBRAPA MEIO-NORTE, under Protocol No. 001/2016.
Pequi oil was obtained from the Governador Alberto Silva school plant at the Federal University of Piauí through transesterification. The process used 10 g of refined pequi oil, 2 g of methanol, and 0.05 g of NaOH. The mixture was stirred at room temperature for 30 minutes, and the reaction was monitored based on the color change of the mixture, following the Soxhlet method as described by AOCS Bc 3-49(8), expressed as a percentage. The mixture was then transferred to a 250-mL separating funnel to separate the esters (upper phase) from the glycerin (lower phase). The upper phase was washed with several 50-mL portions of distilled water to remove impurities and then heated to 110°C to dry the esters (biodiesel).
Volatile constituents were analyzed using gas chromatography–mass spectrometry (GC-MS). The equipment included a Thermo Scientific GC Ultra with a Thermo Scientific SHIMADZU GC-17A ISQ flame ionization detector coupled to a Thermo Scientific QP5050A ISQ mass spectrometer, equipped with an Agilent DB-5 HT HP-5MS capillary column.
The Tris-egg yolk diluent was prepared with 3.605 g of Tris, 2.024 g of citric acid, 1.488 g of fructose, 25 mg of gentamicin, 50,000 IU of penicillin, 100 mL of distilled water, 20% egg yolk, and 5% glycerol, resulting in an osmolality of approximately 350 mOsm and a pH of 6.8. This preparation was conducted at the Animal Reproduction Biotechnology Laboratory of the Agricultural Sciences Center at the Federal University of Piauí and was used for semen dilution and freezing. Four experimental diluents were prepared by adding 0.0, 0.5, 1.0, and 1.5 mL/L of pequi ester to the Tris-egg yolk diluent.
Six Curraleiro Pé Duro bulls from the Brazilian Agricultural Research Corporation (EMBRAPA Meio Norte), located in Campo Maior, Piauí, Brazil (04°49’40” S latitude and 42°10’07” W longitude), were used. The bulls had an average age of 5 years, a weight range between 310 and 365 kg, and a body condition score of 3 to 4 (on a scale of 1 to 5). All bulls had a history of proven fertility and were evaluated for general health, reproductive organ integrity, and semen quality. During the experiment, the bulls were kept in an extensive system with free access to Panicum maximum paddocks, water, and mineral salt.
Semen samples were collected once a week for 7 weeks, resulting in a total of 42 ejaculates, using electroejaculation (Biocon®; Uberaba, Minas Gerais, Brazil) with a sterile 15-mL graduated test tube protected with aluminum foil to prevent light exposure. The ejaculates were transported in an insulated container at 37°C to the Animal Reproduction Laboratory at the EMBRAPA Experimental Field, Campo Maior, for evaluation. Upon arrival, the semen samples from each animal were placed in a water bath at 37°C and individually evaluated for color, appearance, volume, total motility, and vigor using a phase contrast microscope (Olympus Ltd., Tokyo, Japan), following the guidelines in the Manual for Andrological Examination and Evaluation of Animal Semen by the Brazilian College of Animal Reproduction(9).
The sperm concentration was measured using a Neubauer chamber at a dilution ratio of 1:200 in a sodium citrate solution containing 4% formaldehyde. Sperm morphology was assessed using the wet chamber method, in accordance with the same manual’s recommendations(9). Only ejaculates with total motility of ≥80%, vigor of ≥3, sperm concentration of ≥3.5 × 109 sperm/mL, and sperm abnormalities of ≤20% were included in the study. Approved samples from the six ejaculates were pooled to increase semen volume and minimize variability among the samples. The pooled semen was then divided into four aliquots, which were maintained at 37°C in a water bath before dilution with the experimental diluents.
Four aliquots of previously evaluated and approved semen were diluted in Tris–egg yolk medium (37°C) containing pequi ester at concentrations of 0.5, 1.0, and 1.5 mL/L, while one aliquot without supplementation served as the control. The diluted semen was placed into 0.25-mL straws, with a final concentration of 160 × 106 sperm/mL, and frozen using a TK 3000® machine (TK Tecnologia em Congelação Ltda, Uberaba, MG, Brazil). The cooling curve was programmed to lower the temperature at a rate of 0.25°C/min until it reached 5°C, where the straws were held for 4 hours.
For freezing, the curve was set to a rate of –20°C/min until the temperature reached –120°C. Immediately afterward, the straws were immersed in liquid nitrogen, organized in racks, and stored in a cryogenic cylinder at –196°C until thawing. Post-cryopreservation analyses were conducted atthe Animal Reproduction Biotechnology Laboratory of the Federal University of Piauí, where the samples were thawed in a water bath at 37°C for 30 seconds. They then underwent the TTR, assessment of sperm membrane functionality, assessment of in vitro embryo production, and quantification of reduced GSH and MDA.
A previously described physiological evaluation method(10) was used to assess sperm thermoresistance. Thawed samples were incubated in a water bath at 37°C for 3 hours and evaluated over time for total motility (MT, %) and sperm vigor (scale of 1–5) using phase contrast microscopy (Olympus Optical Co., Ltd., Tokyo, Japan) at 400× magnification. Assessments were conducted at 0, 60,120, and 180 minutes(10).
Sperm membrane functionality was assessed using the HOST. For this, 20 μL of semen was incubated at 37°C for 60 minutes in a 150-mOsm/kg solution containing D-fructose (13.5 g/L; Sigma) and sodium citrate (7.3 g/L; Sigma)(11). After the incubation period, the samples were fixed in 0.5 mL of formalin saline. Then, 10 μL of the solution was placed on a slide and covered with a coverslip. Subsequently, 100 sperm were classified according to the presence or absence of tail folding/coiling under light microscopy (1000× magnification). The osmotic response was calculated as % HOST = (% of changes in the tail region after HOST) – (% of changes in the tail region of the spermatozoa before HOST)(12,13).
Fertility was assessed through in vitro embryo production(14). Ovaries were collected from slaughterhouse cows and transported to the laboratory at 35°C in a thermal container containing DMPBS-FLUSH (Nutricell®). In the laboratory, the ovaries were washed with DMPBS-FLUSH at 35°C, and the cumulus-oocyte complexes (COCs) were recovered by aspirating 2- to 8-mm follicles using disposable 21G needles connected to a 10-mL syringe.
The contents of the follicular aspirate were deposited in a 100- × 20-mm Petri dish for scanning under a stereomicroscope. The selected COCs were transferred to a 100- × 20-mm Petri dish containing TQC Holding Plus maintenance medium (Nutricell®) and classified according to morphological quality into Grades I, II, III, and IV(15). Only viable COCs (Grades I and II) were selected for maturation according to the treatments.
The selected COCs underwent three washes in maturation medium in 100- × 20-mm Petri dishes. Ten to 20 oocytes were used per 100 μL of maturation medium (GeneUp Biotechnology), composed of TCM 199 and 10% fetal bovine serum, under mineral oil in 100- × 20-mm Petri dishes. The dishes were incubated at 38.5°C in an atmosphere of 5% CO2 in air for 24 hours. After maturation, the COCs were fertilized in fertilization medium (Gene Up Biotechnology) containing penicillin/hypotaurine/epinephrine, heparin, and bovine serum albumin for 22 hours at 38.5°C in an atmosphere of 5% CO2 in air.
For fertilization, samples corresponding to the control and the treatments of 0.5, 1.0, and 1.5 mL/L of pequi ester were thawed at 37°C for 30 seconds and centrifuged in a Percoll gradient (45% and 90%; GeneUp Biotechnology) at 8000 rpm for 7 minutes. After centrifugation, the supernatant was removed, and the sperm were resuspended in 1 mL of fertilization medium, then centrifuged again at 3200 rpm for 5 minutes. The supernatant was discarded, and 5 μL of the pellet (from a total of 60 μL) was diluted in 250 μL of formaldehyde saline for sperm counting using a Neubauer chamber. An additional 5 μL of the pellet was placed between a slide and coverslip to assess motility and vigor. Each drop was then co-incubated with sperm from the experimental treatments at a final concentration of 1 × 106 sperm/mL.
The presumptive zygotes were washed three times in synthetic oviductal fluid (SOF) medium (Gene Up Biotecnologia®) and then transferred to a 100- × 20-mm Petri dish containing microdrops of 100 μL of SOF medium, composed of SOF, epidermal growth factor), bovine serum albumin, and fetal bovine serum. The dishes were kept in an incubator at 38.5°C in 5% CO2 for 7 days.
The cleavage rate was assessed 48 hours after the start of culture, and blastocyst formation was evaluated 168 hours later according to a previously described method(16).
MDA quantification was performed using the thiobarbituric acid (TBA) method, as previously described(17). MDA levels were measured after supplementing 500 μL of post-cryopreserved semen with Tris-citric acid buffer (pH 7.4) and adding 1 mL of TBA reagent (comprising 15% trichloroacetic acid, 0.25 N hydrochloric acid, and 0.375% TBA) and 1% (v/v) 50 mM butylated hydroxytoluene. The mixture was heated in boiling water (100°C) for 15 minutes. The samples were then cooled and centrifuged at 1,200 × g for 15 minutes. The supernatant was collected, and absorbance was measured at 532 nm using an ultraviolet-visible spectrophotometer (Lambda 25; PerkinElmer). The concentration of MDA was determined using a calibration curve prepared daily with MDA standards at concentrations ranging from 1 to 20 μM. The MDA levels were expressed as nmol of TBA reactive substances per mL of diluent.
The quantification of reduced GSH was based on the Ellman reaction using 5,5’-dithiobis (2-nitrobenzoic acid) (DTNB), with the method modified as previously described(18). In total, 400 μL of the samples was added to a tube containing EDTA buffer (pH 5.4), followed by 320 μL of distilled water and 80 μL of 50% trichloroacetic acid. The mixture was shaken and centrifuged at 3000 rpm for 15 minutes. Then, 400 μL of the supernatant was collected and combined with 800 μL of 0.4 M Tris-HCI buffer (pH 8.9) and 20 μL of 0.01 M DTNB. After 1 minute of reaction, the absorbance was read at 412 nm using a spectrophotometer. The concentration was expressed in μM/mL. For the GSH standard curve, GSH solutions were prepared at concentrations of 6.66, 13.33, 26.66, 40, 53.33, and 66 μM.
The experimental design was a randomized block with four treatments (control and 0.5, 1.0, and 1.5 mL/L), six blocks (animals), and seven repetitions (collections). The TTR and evaluation of sperm membrane functionality after thawing were subjected to analysis of variance (ANOVA) using the general linear models procedure (Proc GLM). The Student– Newman–Keuls (SNK) test was applied to compare the means, with a significance level of 5%. The analyses were performed using the Statistical Analysis System software (SAS Institute Inc., 2013).
The cleavage rate and blastocyst production were evaluated using the chi-squared test with a 5% level of significance. The quantification of reduced GSH and MDA was analyzed using ANOVA, followed by Tukey’s post hoc test at a 5% significance level. The analyses were performed using GraphPad Prism 6.01 software (GraphPad Software, USA, 2012).
3. Results
The analysis of the fatty acid profile of pequi oil (C. coriaceum), as shown in Figure 1 and Table 1, revealed a predominant composition of unsaturated fatty acids, with oleic acid being the most abundant, accounting for 46.3% of the total. Stearic acid (29.6%) and palmitic acid (21.5%) were the other major components, while unsaturated fatty acids such as palmitoleic (1.2%) and linoleic (1.4%) were present at very low levels.
The intensity of the fatty acid compounds in pequi biodiesel is illustrated in Figure 1, and the concentrations of these compounds are detailed in Table 1.
The results from the analysis of sperm motility at different incubation times and concentrations of pequi esters (C. coriaceum) are presented in Table 2. There was a significant decrease in sperm motility over time (P < 0.05); however, no statistical differences were observed between the treatments (P > 0.05). At the initial time point (0 minutes), all treatments showed similar motility values, with the control group and the pequi ester-treated groups displaying average motility between 23.71% and 29.52%. By the end of the 180-minute incubation period, sperm motility had decreased markedly, with the control group showing only 5.95% motility, while the pequi ester-treated groups exhibited slight improvements, particularly the 0.5 mL/L treatment, which achieved 9.02%.
Total post-thaw motility of bovine semen cryopreserved in three different concentrations (0.5, 1.0, and 1.5 mL/L) of pequi ester (C. coriaceum) assessed by the physiological TTR
The data in Table 3 show a progressive and significant reduction in vigor over time (P < 0.05); however, no statistical differences were observed between the treatments (P > 0.05.
Post-thawing vigor of bovine semen cryopreserved in three different concentrations (0.5, 1.0, and 1.5 mL/L) of pequi ester (C. coriaceum) assessed by the physiological TTR
The treatments with different concentrations (0.5, 1.0, and 1.5 mL/L) of pequi ester showed averages similar to the control, ranging from 36.54 to 38.61, with no significant difference (P > 0.05) between treatments in terms of plasma membrane functionality (Table 4).
Functionality of the plasma membrane after thawing bovine semen cryopreserved in three different concentrations (0.5, 1.0, and 1.5 mL/L) of pequi ester (C. coriaceum)
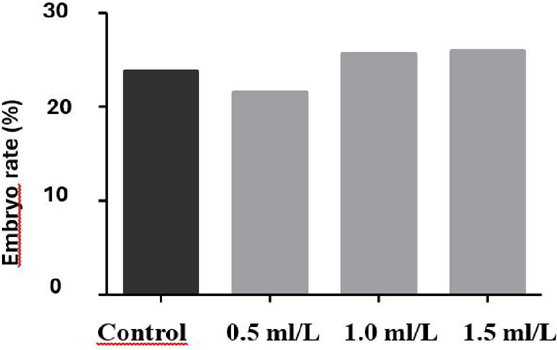
The cleavage rate and embryo production rate evaluated during in vitro production did not differ significantly between treatments (P > 0.05) (Figures 2 3).
Cleavage rate of oocytes fertilized with bovine semen cryopreserved in three different concentrations (0.5, 1.0, and 1.5 mL/L) of pequi ester (C. coriaceum), and a control group with no experimental treatment. The values represent the mean ± SEM. Differences between the groups were determined by ANOVA.
Embryonic development rate of oocytes fertilized with bovine semen cryopreserved in three different concentrations (0.5, 1.0, and 1.5 mL/L) of pequi ester (C. coriaceum), and a control group without experimental treatment. The values represent the mean ± SEM. Differences between the groups were determined by ANOVA.
The quantification of reduced GSH (Figure 4) in cryopreserved bovine semen did not differ significantly between treatments (P > 0.05). However, the quantification of MDA in bovine semen (Figure 5) after thawing showed a significant difference (P < 0.05), with a reduction in MDA concentration observed in the 1.0- and 1.5-mL/L treatments compared with the 0.5-mL/L treatment of pequi ester and the control.
Quantification of reduced GSH in bovine semen cryopreserved with three different concentrations (0, 0.5, 1.0, and 1.5 mL/L) of pequi ester (C. coriaceum) and a control group without experimental treatment. The values represent the mean ± SEM. Differences between groups were determined by ANOVA.
Quantification of MDA in bovine semen cryopreserved with three different concentrations (0.5, 1.0, and 1.5 mL/L) of pequi ester (C coriaceum) and a control group with no experimental treatment. The values represent the mean ± SEM. Differences between the groups were determined by ANOVA. Means with distinct capital letters in the column differ (P < 0.05) according to the SNK test.
4. Discussion
Oleic acid (18:1) is a monounsaturated fatty acid widely recognized for its antioxidant and health-promoting properties. Its presence in high concentrations can help protect against oxidative stress, which is important for preserving sperm quality during cryopreservation. Studies have demonstrated that supplementation with unsaturated fatty acids can improve sperm plasma membrane integrity and reduce oxidative damage(19).
Stearic acid (18:0) and palmitic acid (16:0), both saturated fatty acids, also play important roles in the lipid composition of the cell membrane. Although a high concentration of saturated fatty acids can adversely affect the fluidity of the sperm membrane, maintaining a balance between saturated and unsaturated fatty acids is crucial for preserving sperm functionality(20). The combination of these fatty acids in pequi oil suggests a synergistic potential that could be utilized to optimize semen preservation.
Additionally, the low concentration of polyunsaturated fatty acids, such as linoleic acid (18:2), is beneficial because these compounds are more susceptible to oxidation, which could compromise semen quality during cryopreservation(21). The lower amount of palmitoleic acid also indicates that pequi oil may have a composition that is less prone to oxidative degradation.
Studies on the use of pequi ester in sperm cryopreservation have not been previously reported. However, studies involving compounds present in pequi ester, such as oleic acid and linoleic acid in the dilution medium, have been shown to enhance the motility of porcine spermatozoa(22). Therefore, it was hypothesized that pequi ester supplementation would increase sperm motility and vigor compared with the control because antioxidants are known to neutralize or reduce the impact of free radicals generated by cellular metabolism(23). Nevertheless, the antioxidant activity of pequi ester in this study did not demonstrate an ability to increase or preserve sperm motility and vigor.
This outcome may be attributed to the antioxidant properties of pequi oil, which, while capable of neutralizing oxidative stress (a factor contributing to decreased motility) did not suffice in maintaining these parameters(21). The reduction in sperm motility over time is an anticipated phenomenon in cryopreservation, where a decline in cell viability is commonly observed(22). The pronounced decline at 120 and 180 minutes indicates the fragility of sperm under non-optimal conditions, even with the presence of antioxidants.
The functionality of the sperm membrane did not differ significantly between treatments and the control in this study, although all samples reacted positively to the test. Previous studies have shown that natural antioxidants can enhance resistance to cryogenic stress by protecting membrane structure and function(24).
The different concentrations of pequi ester did not influence the cleavage rate or embryo production rate, although lower MDA production was observed in the pequi ester treatments than in the control. This outcome can be attributed to various factors involved in cryopreservation and embryo development. Recent studies suggest that semen cryopreservation not only affects the integrity of the plasma membrane but also causes damage to sperm DNA and mitochondrial function, both of which are crucial for proper embryonic development(24,25). However, these tests were not conducted in this study.
The lack of effect on cleavage rates may be due to the fact that while pequi ester has antioxidant properties, its capacity to prevent lipid peroxidation may not be sufficient to protect against deeper damage, such as sperm DNA fragmentation. DNA fragmentation can occur even in motile sperm, leading to low-quality embryos that fail to progress to more advanced developmental stages(26).
The quantification of reduced GSH in the treatments remained consistent and did not differ from the control. GSH activity plays an important role in controlling the generation of lipid peroxides, preventing changes in plasma membrane fluidity, which is necessary for sperm-oocyte fusion(27). However, cryopreservation can alter sperm metabolism by inducing an imbalance, increasing the production of reactive species, and reducing the antioxidant activity of semen, ultimately decreasing GSH peroxidase levels(28). Another study that evaluated the effect of pequi pulp in the diet of rats showed a protective effect on the liver, increasing total GSH content and reducing lipid peroxidation(29).
In this study, the addition of 1.0 and 1.5 mL/L of pequi ester (C. coriaceum) was effective in preventing the production of MDA, a marker of lipid peroxidation. This finding aligns with other studies demonstrating that antioxidants can inhibit lipoperoxidation during sperm cryopreservation(24,25). Oxidative stress, caused by an imbalance between the production of ROS and the cell’s antioxidant capacity, is a primary cause of damage to cell membranes and reduced sperm motility during cryopreservation(25).
Mitochondria are especially susceptible to ROS-induced damage because they are the main sources of ROS production within cells. When compromised, they contribute to oxidative stress, resulting in severe damage to sperm membranes, DNA, and ultimately sperm immobilization(24). The addition of antioxidants, such as pequi ester, aims to neutralize ROS and minimize their negative impact on cell integrity and function during the freezing and thawing processes(25).
Previous studies have shown that pequi pulp possesses significant antioxidant potential, attributed to its high levels of ascorbic acid (vitamin C)(18). In vivo, ascorbic acid is an effective antioxidant capable of neutralizing ROS and inhibiting lipid peroxidation, either directly or indirectly. It effectively scavenges ROS such as O2++, H2O2, hypochlorite (CIO-), OH-, and peroxyl radicals (OOH˙)(30). However, under certain conditions, vitamin C can act as a pro-oxidant, stimulating the production of H2O2 and promoting Fenton reactions, which generate hydroxyl radicals (OH+). In excess, these radicals can also cause cellular damage(25).
5. Conclusion
Based on the findings, it can be concluded that supplementation with pequi ester (1.0 and 1.5 mL/L) in the cryopreservation of bovine semen may be beneficial for minimizing the effects of lipid peroxidation in sperm, as evidenced by the reduction in MDA concentration after thawing.
Acknowledgments
The authors would like to thank EMBRAPA MEIO-NORTE for providing the animals used in the experiment. The authors also thank CAPES for their financial support.
References
-
1 Sapanidou V, Tsantarliotou MP, Lavrentiadou SN. A review of the use of antioxidants in bovine sperm preparation protocols. Anim Reprod Sci. 2023;251(4):1-11. https://doi.org/10.1016/j.anireprosci.2023.107215
» https://doi.org/10.1016/j.anireprosci.2023.107215. -
2 Durairajanayagam D. Physiological role of reactive oxygen species in male reproduction. In: Henkel R, Samanta L, Agarwal A, editors. Oxidants, antioxidants and impact of the oxidative status in male reproduction. 1st ed. Academic Press; 2019. p. 65-78. https://doi.org/10.1016/B978-0-12-812501-4.00008-0
» https://doi.org/10.1016/B978-0-12-812501-4.00008-0. -
3 Baldi E, Tamburrino L, Muratori M, Degl’Innocenti S, Marchiani S. Adverse effects of in vitro manipulation of spermatozoa. Anim Reprod Sci. 2020;220:106314. https://doi.org/10.1016/j.anireprosci.2020.106314
» https://doi.org/10.1016/j.anireprosci.2020.106314. -
4 Pintus E, Ros-Santaella JL. Impact of oxidative stress on male reproduction in domestic and wild animals. Antioxidants. 2021;10(7):1154. https://doi.org/10.3390/antiox10071154
» https://doi.org/10.3390/antiox10071154. -
5 Lima A, Silva AMO, Trindade RA, Torres RP, Mancini-Filho J. Composição química e compostos bioativos presentes na polpa e na amêndoa do pequi (Caryocar brasiliense, Camb.). Revista Brasileira de Fruticutura. 2007;29(3):695-698. https://doi.org/10.1590/S0100-29452007000300052
» https://doi.org/10.1590/S0100-29452007000300052. -
6 Polewski K, Kniat S, Slawinska D. Gallic acid, a natural antioxidant, in aqueous and micellar environment: spectroscopic studies. Curr Top Biophys. 2002;26(2):217-227. https://www.researchgate.net/publication/284542596_Gallic_acid_a_natural_antioxidant_in_aqueous_and_micellar_environment_spectroscopic_studies
» https://www.researchgate.net/publication/284542596_Gallic_acid_a_natural_antioxidant_in_aqueous_and_micellar_environment_spectroscopic_studies -
7 Miranda -Vilela AL, Akimoto AK, Alves PCZ, Pereira LCS, Gonçalves CA, Klautau-Guimarães MN, et al. Dietary carotenoid-rich pequi oil reduces plasma lipid peroxidation and DNA damage in runners and evidence for an association with MnSOD genetic variant -Val9Ala. Genet Mol Res. 2009;8:1481-1495. https://doi.org/10.4238/vol84gmr684
» https://doi.org/10.4238/vol84gmr684. -
8 American Oil Chemists’ Society. Official methods and recommended practices of the American Oil Chemists’ Society. 4th ed. Champaign (IL): American Oil Chemists’ Society; 1993. https://library.aocs.org/
» https://library.aocs.org/ -
9 CBRA – Colégio Brasileiro de Reprodução Animal. Bovinos. Manual para exame andrológico e avaliação de sêmen animal. 3. ed. CBRA. Belo Horizonte-MG, p. 47-50, 2013. Disponível em: http://cbra.org.br/br/
» http://cbra.org.br/br/ -
10 Vianna FP, Papa FO, Zahn FS, Melo CM, Dell’Aqua Jr JA. Thermoresistance sperm tests are not predictive of potential fertility for cryopreserved bull semen. Anim Reprod Sci. 2009;113:279-282. https://doi.org/10.1016/j.anireprosci.2008.06.009
» https://doi.org/10.1016/j.anireprosci.2008.06.009. -
11 Correa JR, Zavos PM. The hypoosmotic swelling test: its employment as an assay to evaluate the functional integrity of the frozen-thawed bovine sperm membrane. Theriogenology.1994;42(2):351-360. Doi: https://doi.org/10.1016/0093-691x(94)90280-1
» https://doi.org/10.1016/0093-691x (94)90280-1 -
12 Melo MIV, Henry M. Teste hiposmótico na avaliação da viabilidade do sêmen equino resfriado com diferentes diluidores. Arquivo Brasileiro de Medicina Veterinária e Zootecnia. 2005;57(6):757-763. https://doi.org/10.1590/S0102-09352005000600009
» https://doi.org/10.1590/S0102-09352005000600009 -
13 Vasconcelos AB, Zandonaide JBZ, Sobrinho ALF, Silva BO, Quintal PNQ. A comparative study of three different dyes evaluating the physical integrity of the plasma membrane of cryopreserved bovine spermatozoa. Veterinária Notícias. 2017;23(1 ):13-22. http://dx.doi.org/10.14393/VTV-v23n1-2017.2
» https://doi.org/10.14393/VTV-v23n1-2017.2. -
14 Oliveira CS, Serapião RV, Quintão CCR. Biotécnicas da reprodução em bovinos: minicursos ministrados durante o 3º Simpósio “Biotécnicas da Reprodução em Bovinos” no Laboratório de Reprodução Animal do Campo Experimental Santa Mónica. Juiz de Fora: Embrapa Gado de Leite; 2014. 52 p. (Embrapa Gado de Leite. Documentos, 175). http://www.infoteca.cnptia.embrapa.br/infoteca/handle/doc/1001858
» http://www.infoteca.cnptia.embrapa.br/infoteca/handle/doc/1001858 -
15 Leibfried L, First NL. Characterization of bovine follicular oocytes and their ability to mature in vitro. J Anim Sci. 1979;48(1):76-86. https://doi.org/10.2527/jas1979.48176x
» https://doi.org/10.2527/jas1979.48176x. -
16 Bó GA, Mapletoft RJ. Evaluation and classification of bovine embryos. Animal Reproduction. 2013; 10(3):344-348. https://www.animal-reproduction.org/article/5b5a604cf7783717068b46a2
» https://www.animal-reproduction.org/article/5b5a604cf7783717068b46a2 -
17 Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 1978; 52:302-10. doi: https://doi.org/10.1016/S0076-6879(78)52032-6
» https://doi.org/10.1016/S0076-6879(78)52032-6. -
18 Machado, M.T.C.; Mello, B.C.B.S.; Hubinger, M.D. Study of alcoholic and aqueous extraction of pequi (Caryocar brasiliense Camb.) natural antioxidants and extracts concentration by nanofiltration. Journal of Food Engineering, V.117, p.450- 457, 2013. https://doi.org/10.1016/j.jfoodeng.2012.12.007
» https://doi.org/10.1016/j.jfoodeng.2012.12.007 -
19 Barros, F N. Efeito da adição do ácido araquidônico e do ácido oleico ao diluidor tris-gema sobre a viabilidade espermática póscongelação em sêmen caprino. 2021. 88 f. Tese (Doutorado em Ciência Animal) – Universidade Federal do Piauí. 2021. http://repositorio.ufpi.br:8080/handle/123456789/2814
» http://repositorio.ufpi.br:8080/handle/123456789/2814 -
20 Ferramosca A, Zara V. Diet and male fertility: The impact of nutrients and antioxidants on sperm energetic metabolism. International Journal of Molecular Sciences. 2022; 25;23(5):2542. doi: https://doi.org/10.3390/ijms23052542
» https://doi.org/10.3390/ijms23052542. -
21 Beygi Z, Forouhari S, Mahmoudi E, Hayat SMG, Nourimand F. Role of oxidative stress and antioxidant supplementation in male fertility. Current Molecular Medicine. 2021;21(4):265-282. doi: https://doi.org/10.2174/1566524020999200831123553
» https://doi.org/10.2174/1566524020999200831123553 -
22 Hossain MDS, Tareq KMA, Hammano K, Tsujii H. Effect of fatty acids on boar sperm motility viability and acrosome reaction. Reproductive Medicine and Biology. 2007; 6: 235-239. doi: https://doi.org/10.1111/j.1447-0578.2007.00191.x PMID: 29699281; PMCID: PMC5904605.
» https://doi.org/10.1111/j.1447-0578.2007.00191.x. -
23 Almeida J, Brito M, Neves BP, Becerra VAB, Auler PA, Haddad JP, Baruselli PS, Henry M. Use of cooled buffalo semen as a strategy to increase conception rates in fixed-time artificial insemination programs during unfavorable reproductive periods. Arquivo Brasileiro de Medicina Veterinária e Zootecnia (online). 2021;73(3): 560-570. https://doi.org/10.1590/1678-4162-12142
» https://doi.org/10.1590/1678-4162-12142 -
24 Khan IM, Cao Z, Liu H, Khan A, Rahman SU, Khan MZ, Sathanawongs A, Zhang Y. Impact of Cryopreservation on Spermatozoa Freeze-Thawed Traits and Relevance OMICS to Assess Sperm Cryo-Tolerance in Farm Animals. Frontiers in Veterinary Science. 2021; 8; 1-14. doi: https://doi.org/10.3389/fvets.2021.609180
» https://doi.org/10.3389/fvets.2021.609180. -
25 Ugur MR, Abdelrahman AS, Evans HC, Gilmore AA, Hitit M, Arifiantini RI, Purwantara B, Kaya A, Memili E. Advances in Cryopreservation of Bull Sperm. Frontiers in Veterinary Science. 2019; 1-15. https://doi.org/10.3389/fvets.2019.00268
» https://doi.org/10.3389/fvets.2019.00268. -
26 Zhang B, Wang Y, Wu1 C, Qiu S, Chen X, Cai B, Xie H. Freeze-thawing impairs the motility, plasma membrane integrity and mitochondria function of boar spermatozoa through generating excessive ROS. BMC Veterinary Research. 2021;17;127: 1-9. doi: https://doi.org/10.1186/s12917-021-02804-1
» https://doi.org/10.1186/s12917-021-02804-1. -
27 Aitken J, Fisher H. Reactive oxygen species generation and human spermatozoa: the balance of benefit and risk. Bioessays. 1994 Apr; 16(4): 259-67. doi: https://doi.org/10.1002/bies.950160409 PMID: 8031303.
» https://doi.org/10.1002/bies.950160409 -
28 Bilodeau JF, Chatterjee S, Sirard MA, Gagnon C. Levels of antioxidant defenses are decreased in bovine spermatozoa after a cycle of freezing and thawing. Mol Reprod Dev. 2000 Mar;55(3):282-8. doi: https://doi.org/10.1002/(SICI)1098-2795(200003)55:3<282::AID-MRD6>3.0.CO;2-7
» https://doi.org/10.1002/(SICI)1098-2795(200003)55:3<282::AID-MRD6>3.0.CO;2-7 -
29 Viana, A. M. F. Efeito da polpa de pequi (Caryocar brasiliense Cambess) nas alterações hepáticas induzidas pela dieta hiperlipídica em ratos. 2015. 108 f. Dissertação (Mestrado em Saúde e Nutrição) - Escola de Nutrição, Universidade Federal de Ouro Preto, Escola de Nutrição, Ouro Preto, 2015. https://www.repositorio.ufop.br/items/248df98a-f4eb-435b-b53b-1b8155199115
» https://www.repositorio.ufop.br/items/248df98a-f4eb-435b-b53b-1b8155199115 -
30 Vasconcelos SML, Goulart MOF, Moura JBF, Manfredini V, Benfato MS, Kubota LT. Espécies reativas de oxigênio e de nitrogênio, antioxidantes e marcadores de dano oxidativo em sangue humano: principais métodos analíticos para sua determinação. Química Nova. 2007; 30(5), 1323-1338. https://doi.org/10.1590/S0100-40422007000500046
» https://doi.org/10.1590/S0100-40422007000500046

 Antioxidant potential of pequi esters (Caryocar coriaceum) in bovine semen cryopreservation and in vitro fertilization
Antioxidant potential of pequi esters (Caryocar coriaceum) in bovine semen cryopreservation and in vitro fertilization