

Abstract
Conjugated linoleic acid (CLA) is a mixture of positional isomers of linoleic acid found in meat and dairy products from ruminants. It is a trans fat widely used by athletes as a food supplement, due to a supposed effect of maximizing the use of body fat reserves. The interest in diet and culture media supplementation with CLA is an emerging area, demanding studies in order to elucidate its benefits in the reproductive parameters, as well as in cryopreservation. Therefore, the aim of this review was to discuss the effects of CLA on the oocytes, sperm and embryos cryotolerance. Some studies have already demonstrated its use in cryopreservation of germline. Among those, it was observed that CLA supplementation during oocyte in vitro maturation can increase their viability post-freezing and developmental capacity. Regarding the use of CLA on sperm, there are few studies and their results are still inconclusive. Finally, studies about CLA supplementation on embryo culture media have shown promising results, indicating that this bioactive molecule is able to modulate lipid uptake on blastomeres. Altogether, these findings demonstrate the potential use of CLA as a bioactive molecule to improve germline and embryo cryotolerance and open new perspectives on human and animal reproduction field.
Keywords: lipid accumulation, cryopreservation, embryo, oocyte, sperm.
Resumo
O ácido linoleico conjugado (CLA) é uma mistura de isômeros posicionais do ácido linoleico encontrado em carne e laticínios de ruminantes. É um tipo de gordura trans muito utilizada por atletas para como suplemento alimentar devido a um suposto efeito de maximizar a utilização das reservas de gordura corporal. O interesse na suplementação de dietas e meios de cultura com o CLA é uma área emergente, exigindo estudos para elucidar seus benefícios nos parâmetros reprodutivos e na criopreservação. Dessa forma, o objetivo dessa revisão foi discutir os efeitos do CLA na criotolerância de oócitos, espermatozóides e embriões. Alguns estudos já demonstraram seu uso na criopreservação da linhagem germinativa. Entre esses, foi observado que a suplementação de CLA durante a maturação in vitro de oócitos pode aumentar sua viabilidade pós-congelamento e capacidade de desenvolvimento. Em relação ao uso de CLA no esperma, existem poucos estudos e seus resultados ainda são inconclusivos. Por último, estudos sobre a suplementação de CLA em meios de cultura de embriões mostraram resultados promissores, indicando que essa molécula bioativa é capaz de modular a captação de lipídios em blastômeros. No total, essas descobertas demonstram o potencial uso do CLA como uma molécula bioativa para melhorar a linha germinativa e a criotolerância ao embrião e abrir novas perspectivas no campo da reprodução humana e animal.
Palavras-chave: acúmulo lipídico, criopreservação, embrião, oócito, sêmen.
Section: Medicina Veterinária
Received
May 22, 2020.
Accepted
August 6, 2020.
Published
October 20, 2020.
www.revistas.ufg.br/vet
visit the website to get the how to cite in the article page.
Introduction
Cryopreservation is a process that preserves organelles, cells, tissues, or any other biological constructs by cooling the samples to very low temperatures (1). It occurs because the biological metabolism in living cells decreases dramatically at low temperatures, which enables the long-term preservation of living cells and tissues for scientific research or for many medical applications. However unprotected freezing is normally lethal (2). A major challenge for cells during this process is not the low temperatures (below -180 °C) during the storage; contrarily, is the lethality of an intermediate temperature zone (-15 to -60 °C) that a cell must pass through twice – during cooling and heating(3). The speeds of cooling and thawing can largely affect physicochemical and biophysical reactions, affecting the survival rate.
Moreover, cryogenic lesions involves osmotic rupture, caused by high concentrations of solutes and the formation of extra and intracellular ice crystals (2). To mitigate these harmful effects, cryoprotectants are normally used in order to increase the total concentration of all solutes in the system and reduce the amount of ice formed at any temperature. To be biologically acceptable, cryoprotectants must be able to penetrate cells and have low toxicity (1,2). Many compounds have these properties, including glycerol, dimethyl sulfoxide, ethanediol, and propanediol. Regardless of the cryopreservation technique, whether it allows freezing (conventional cryopreservation) or preventing freezing (vitrification), the cryoprotectant must access all cell components (2). However, barriers such as cell membranes and intracytoplasmic lipid droplets, compromise diffusion and osmosis, interfering with the introduction and removal of cryoprotectants during freezing and thawing. Thus, the modulation of membrane properties and the amount of intracellular lipids can improve the efficiency of cell survival during the cryopreservation process.
In this regard, studies about diet and in vitro culture media supplementation with fatty acids, such as conjugated linoleic acid, have been used as a strategy to modulate the composition of the membrane and the amount of intracytoplasmic lipids, aiming to improve the efficiency of cryopreservation of gametes (4, 5) and embryos (6, 7). Considering this scenario, the aim of this review is to provide a state of art about the use of CLA on germline and embryo cryopreservation, describing the main findings published in this field.
1. Conjugated linoleic acid (CLA) biological synthesis
Conjugated linoleic acid (CLA) refers to a mixture of linoleic acid positional and geometric isomers, characterized by having conjugated double bonds, not separated by a methylene group as in linoleic acid. These double bonds are usually located at positions 8 and 10, 9 and 11, 10 and 12, 11 and 13, and can occur in cis-cis, trans-cis, cis-trans and trans-trans configuration (8). Among all possible combinations with these characteristics, only two have proven bioactivity (cis-9, trans-11 CLA, and trans-10, cis-12 CLA), reducing carcinogenesis (9), anti-obesity effect (10, 11, 12), changing the lipid composition of bovine milk (13), affecting positively on diabetes mellitus, as well as improving immune response (14). These isomers can be synthesized in the rumen, adipose tissue and mammary gland, in a process known as endogenous synthesis.
1.1. Ruminal synthesis
The synthesis of CLA in the rumen occurs through incomplete biohydrogenation of polyunsaturated fatty acids from the diet by ruminal microorganisms (15). This event requires prior lipolysis of fatty acids esterified in the form of triglycerides, phospholipids and galactolipids, by microbial lipases present in the rumen. The unsaturated free fatty acids from lipolysis are then subjected to biohydrogenation. During the biohydrogenation process of linoleic acid, the cis-9, trans-11 isomer is the first intermediate formed by ruminal bacteria. The Δ12 cis, Δ11 trans isomerase enzyme catalyzes the isomerization of linoleic acids to cis-9, trans-11 CLA, which is saturated at the position of the cis-9 double bond by the reductase enzyme, forming vaccenic acid (C18:1). This reductase enzyme needs free carboxyl radicals (COOH) to complete the reaction, which requires prior lipolysis of lipids from the diet. The next step involves a subsequent reduction from vaccenic acid (C18:1) to stearic acid (18:0) (16, 17). However, during this event, the intermediaries of this process can pass through the rumen, move through the bloodstream and be absorbed and incorporated into the fat in the tissues.
Griinari and Bauman(18) proposed that the isomer of cis-9, trans-11 CLA, can eventually be converted into C18:1 trans-10 in rumen content. This speculation about the production of trans-10, cis-12 CLA was confirmed later. Coakley et al. and Ando et al., demonstrated that the Bifidobacterium sp, Propionibacterium sp, Lactococcus sp, Streptococcus sp and Lactobacillus sp, isolated from other habitats can also produce trans-10, cis-12 CLA (19, 20). These bacteria can be found in the rumen, although in a very small number, contributing to bio-hydrogenation and formation of trans-10, cis-12 CLA. The population of these microorganisms was significantly increased in the rumen of animals feed with concentrated diets, which is consistent with the higher production of trans-10, cis-12 CLA (21). According to Griinari et al. (22) and Chouinard et al.(23) there are some conditions that can intensify ruminal CLA synthesis, such as changes in the ruminal environment, increments in the amount of fatty acids in the diet and modifications in ruminal pH.
1.2. Non-ruminal synthesis
Endogenous CLA synthesis in tissues begins when C18:1 fatty acid is desaturated by the enzyme Δ9 desaturase (stearoyl-CoA desaturase – SCD), present in the mammary gland and in adipose tissue (24). In animals, desaturation occurs only up to carbon 9, due to the absence of enzymes Δ12 and Δ15 desaturases, present only in vegetables. Consequently, linoleic acid is considered an essential fatty acid and must be supplied through the diet as it is an essential precursor in the synthesis of prostaglandins. SCD introduces a double bond between carbons 9 and 10 of fatty acids. Reactions catalyzed by desaturases are essential to maintain the cell membranes fluidity (25).
To verify the hypothesis of endogenous CLA synthesis by the SCD enzyme, Griinari et al. (24) infused the abomasum of lactating cows with a mixture of C18:1 trans-11 and C18:1 trans-12 (50% -50%) and observed an increment of 31% in CLA cis-9, trans-11 secreted in the milk. Based on that, they concluded that animals are really capable of endogenously produce cis-9, trans-11 CLA. In the other experiment, evaluating the contribution of endogenous CLA synthesis via SCD, the authors infused sterculia oil (an SCD inhibitor) and estimated that 64% of CLA in ruminants milk is endogenously produced. Corroborating these data, Corl et al. (26) also reported a 60-65% reduction in the cis-9, trans-11 isomer when the animals received a diet supplemented with sterculia oil. Kay et al. (27) estimated that 87 to 100% of the cis-9, trans-11 isomer, present in milk fat, was produced by an endogenous pathway in cows under pasture and supplemented with sterculia oil, they also demonstrated that it is possible to increase the levels of the cis-9, trans-11 isomer in milk, through the supplementation of trans fatty acids C18: 1.
In order to assess the biological effect of CLA on SCD activity, Lee et al. (28) using mice as experimental models, supplementing the diet with 42% of the cis-9, trans-11, and 44% of the trans-10, cis-12 isomer expression. In this study, a relative reduction in the expression of SCD hepatic messenger RNA (mRNA) was observed. In another experiment in which only the cis-9, trans-11 isomer was used, SCD expression was not altered. Based on these data, the authors inferred that the trans-10, cis-12 isomer is responsible for the inhibitory effects of SCD expression, a result confirmed later by Park et al (29). According to these data, it is assumed that trans-10, cis-12 isomer acts by directly inhibiting the SCD and that probably the double bond in the cis-12 position is the most important in this CLA inhibitory effect.
2. CLA modulates intracellular lipids accumulation
Lipid homeostasis in mammalian cells is regulated by a family of transcription factors called sterol response element-binding protein (SREBP). These transcription factors control the activity and expression of more than 30 genes related to the synthesis of cholesterol, fatty acid, triglycerides, and phospholipids (30). In vivo and in vitro studies, reported that trans-10, cis-12 CLA influences the amount of intracellular lipids, through the modulation in gene expression and activity of enzymes under the command of SREBP.
During lipogenesis, the first fatty acid molecule (palmitic acid) is obtained from one molecule of acetyl-CoA and seven of malonyl-CoA. It requires two fundamental enzymes, (1) acetyl-CoA carboxylase (ACC), and (2) fatty acid synthases (FAS), responsible for the synthesis of malonyl-CoA and palmitic acid, respectively. Independent studies (31, 32) demonstrated that the infusion of the trans-10, cis-12 isomer in the bovine abomasum significantly reduced the expression of these lipogenic enzymes, consequently, decreasing milk fat content (48%), tissue lipogenic capacity (82%) and the expression of glycerol phosphate acyltransferase and acyl glycerol phosphate acyltransferase enzymes. Similarly, in in vitro studies, Pereira et al.(6) showed that CLA supplementation in culture media reduced lipid accumulation in bovine embryos. Later, Batista et al. (7) demonstrated that this reduction occurs through the modulation in the expression of 1-acylglycerol 3-phosphate 0-acyltransferase (AGPAT) enzyme involved in triglycerides synthesis. Therefore, CLA supplementation has been proposed as an alternative to down-regulates lipogenic related genes (33), inhibits triacylglycerols synthesis and uptake, and consequently reduces the intracytoplasmic lipid accumulation (34, 35). In despite of all these exciting findings, the mechanisms that control lipid reduction induced by CLA was not yet fully elucidated.
3. CLA regulates cell membrane functionality
Cellular membrane surrounds the cell, limiting and maintaining the differences between cytosol and the extracellular matrix. Within eukaryotic cells, membranes of the nucleus, endoplasmic reticulum, Golgi apparatus, mitochondria, and other membrane-bounded organelles maintain their physicochemical properties, as well as physiology (36). This structure is formed by a lipid bilayer (phospholipids, glycolipids, and sterols) and proteins, playing a central role in their functioning (37). During cryopreservation, intracellular ice crystals can rupture the plasma membrane and release cellular components (38). The incorporation of fatty acids in the phospholipid double layer of the plasma membrane alters its fluidity, and as a consequence can interfere with cellular metabolism (39). The components present in in vitro culture media can lead to lipid accumulation and its excessive content would cause changes in the plasma membrane, such as alterations in fluidity and functions (34), indicating a positive correlation between membrane fluidity and freezing tolerance. These modifications occur mainly due to the changes in the expression of genes related to adipogenesis (40). One of the main effects of CLA supplementation on embryo cryopreservation is the reduction in the expression of enzymes, caused by chemical agents responsible for triglyceride catalysis, reducing lipid exposure in embryonic cells (6). Additionally, Leite et al. (41) proposed that the addition of CLA in the culture medium can affect enzymatic digestion of the zona pellucida, altering the hatching rate of embryos.
Cis-9, trans-11 and trans-10, cis-12 isomers supplementation changes lipidic profile of bovine embryos, reducing lipid droplets accumulation (42). The trans-10, cis-12 isomer acts on the absorption of free fatty acids without increasing lipolysis, on the incorporation of CLA in the lipid bilayer of the embryonic cell membrane, increasing the fluidity of the membrane (6, 42, 43) and consequently increasing the resistance to the cryopreservation.
4. Effect of CLA supplementation on semen cryopreservation
The spermatogenesis is a complex biological process in which a diploid germ cell (spermatogonia) after serial mitotic divisions gives rise to haploid germ cells. These spermatids gradually differentiate in spermatozoa that after spermiation, are released in the seminiferous tubules lumen. These sperm are passively transported to epididymis, where they acquire motility in a process named sperm maturation (44). Morphologically, sperm are divided into head (acrosomal and post-acrosomal) and tail (45). The tail of the male gamete is composed of the neck, main and terminal intermediate piece (46). The plasma membrane is responsible for involving all the sperm components and is composed of lipid and protein layers such as those containing phospholipids, cholesterol, glycolipids, and different types of proteins (47). The lipid composition of the plasma sperm membrane plays an important role in the physiological changes that lead to fertilization, in addition, it also affects the response of the sperm to cooling and freezing (48). This lipid composition on the membrane can be manipulated both In vivo and in vitro.
The species differences in the bull freezability of spermatozoa are in part attributable to the polyunsaturated fatty acid (PUFA) contents of their plasma membrane (49). In this sense, it has been shown that dietary supplementation with a wide range of PUFA supplements can alter the sperm fatty acid profile. Studies with rams (50, 51, 52), bulls (53, 54), fowl, and boars (56) have suggested benefits after dietary supplementation of PUFA on some parameters. Like other polyunsaturated fatty acids, CLA can be incorporated into membrane phospholipids and perform biological effects as well, as demonstrated in the case of omega-3 fatty acids (57). However, using the rabbit as an experimental model, Abdelatty et al. (58) reported that supplementation of CLA (a mixture of the same proportion of isomers c9, t11-CLA and t10, c12-CLA) in the long term can alter the reproductive potential of males, especially if fed at a dose greater than 0.5%. In this study, the authors observed that 1% CLA supplementation decreased sperm motility and progressive motility, in addition to decreasing the testicular concentration of L-carnitine and α-tocopherol. This decrease in the amount of antioxidants in the testis, was associated with increased apoptosis in spermatogonia cells in the seminiferous tubules in the groups treated with CLA.
A similar result was also observed by Karimi et al. (59) in Holstein bulls. Evaluating the effect of CLA supplementation in the diet on the quality and freezability of sperm, the authors did not observe the effect of CLA on semen volume, sperm concentration and total sperm production (p> 0.05). However, the proportion of sperm with abnormal morphology in fresh semen increased significantly (p <0.05) in the CLA-fed group compared to the control group. In addition, in the CLA-fed group, the proportion of post-thaw sperm with abnormal morphology at week 10 of the trial was significantly higher in the CLA than in the control group (p <0.05). Progressive motility tended to increase in the CLA-fed group, although dietary supplementation did not affect other CASA parameters or viability in fresh and thawed sperm (59).
The addition of fatty acids in semen cryopreservation media may influence the sperm motility after thawing, possibly by maintaining the membrane fluidity due to their incorporation in lipid bilayers. Maldjiana et al. (60) reported that the presence of lipids as diluents for cooling and freezing is essential to exchange components in an extracellular environment (61). In ovine semen, the addition of oleic-linoleic acids to the cryopreservation medium resulted in a beneficial effect in the preservation of sperm cell viability (62). Swine spermatozoa incubated for 4 h at 37 ºC in a dilution media containing oleic and linoleic acids demonstrated a significant improvement in motility and viability (63). The use of linoleic acid in the bovine semen cryopreservation medium caused an improvement in sperm motility after thawing, relating this result to a possible maintenance in membrane fluidity due to the incorporation of linoleic acid by the lipid bilayer (64). According to Kaeoket(65), semen extenders supplementation with some fatty acids would be a promising strategy to minimize oxidative oxygen species and also to protect the plasma membrane.
Few studies have been evaluated the impact of CLA in the sperm cryopreservation, Soares et al. (66) showed that the use of CLA isomers (cis-9, trans-11 and cis-10, trans 12) in the dilution medium of bovine sperm did not cause evident changes on viability and motility. However, in the treatment with 50 µM of CLA, sperm showed the highest values of average speed, and they also present a satisfactory fertilization rate (67). Meanwhile, in the treatment with 100 µM of CLA, sperm with a higher percentage of intact membrane and high mitochondrial potential were observed, however, none of these differences were significant (66).
More recently, Teixeira et al. (5) analyzed the use of 50 µM CLA in cryopreservation of wild boar semen, and have not observed advantages on the post-thaw boar sperm viability and integrity. Otherwise, Karimi et al. (59) demonstrated that Holstein bulls that received a CLA supplemented diet showed an increased sperm progressive motility but this change has no significant benefits, however, these authors have not observed any other advantage of this supplementation in both, fresh and frozen/thawed samples. Overall, the data available in the literature regarding CLA semen parameters are inconclusive, demanding more studies.
5. Effect of CLA supplementation on cryopreservation of oocytes
In mammals, germ cells develop surrounded by somatic cells forming ovarian follicles. The ovarian folliculogenesis starts during the embryonic development, since the dormant primordial follicle are activated and grows to the ovulatory follicles, that in the ovulation, releases metaphase II oocytes (68). The lipids present in the oocytes are mostly triacylglycerols stored as lipid drops in the cytoplasm (69). Possibly, oocyte lipid accumulation occurs: (i) increasing lipogenesis, (ii) decreasing beta fatty oxidation and (iii) increasing cholesterol uptake from extracellular matrix or culture medium. According to Baumgard et al.(31) CLA decreases lipids by a downregulation of lipogenic enzymes, and also reducing the levels of enzymes used in the consumption of circulating fats (7).
The demand for women fertility preservation has been increased among the years, mainly because of socioeconomic changes that led to the usual pregnancy postponing. Additionally, the increasing number of childhood cancer diagnostics in non-reproductive age and the improvements in cancer treatments claim for the necessity to preserve child gametes to be used after cancer remission. For female fertility preservation, oocyte cryopreservation would be the best option (70), however, because of their morphological characteristics, mainly due to the oocyte size, their cryopreservation is highly hampered and less efficient when compared to embryo and sperm cryopreservation (71).
Moreover, oocyte cryopreservation is very interesting for all animal species, especially for zootechnical purposes. In past years, some advances had been achieved in this field enhancing oocyte survival after cryopreservation, however, the current protocols are still inefficient. Female gametes are huge cells, with a reduced area/membrane surface rate. Thus, ice crystals setting up during the cryopreservation occurs more frequently, resulting in cell death after thawing. Furthermore, the cryopreservation stress would modify zona pellucida or ooplasm, causing structural and functional damage (72). Besides that, the membrane properties affect water and cryoprotectants flow, making the process even more difficult and sometimes unfeasible (73). Lipid conformation of embryos and oocytes is still considered as a parameter of quality and cryotolerance, mainly because of main damage related to cryopreservation occurs due to membrane or intracellular lipid modifications (74). It is already known that bovine oocytes are more resistant to cryopreservation compared to other investigated mammals' species, and some authors suggested that it is due to their differences in the lipid composition (75). Even in cattle, the difference between breeds is notable, such as the number of cytoplasmic droplets and oocyte competence (76). Aardema et al. (77) noted that fatty supplementation in in vitro culture medium, such as linoleic acid, has positive effects on oocyte maturation, development of blastocysts, and increased tolerance to the cryopreservation of bovine oocytes. Ferreira et al. (78) suggested that the quality control of in vitro culture media is relevant to the understanding of cryopreservation processes.
In 2011, Lapa et al. (4) studied the effect of CLA in oocyte maturation and in the lipid composition of cumulus-oocyte complexes (COCs). They did not observe significant differences in maturation or in embryo production rates. On the other hand, oocyte maturation in media supplemented with CLA (100 mM) led to a higher number of embryos with better quality at day 8, compared to the control group (7.7 ± 3.3 to 0.0 ± 0.0 with very good quality and 32.2 ± 5.7 to 23.1 ± 7.4 with good quality, respectively). Consequently, this finding indicates that the maturation environment has an important influence in the oocyte capacity to generate healthy embryos with the ability to reach more advanced levels of development, and ultimately, that CLA supplementation on this stage, would modulate oocyte energy metabolism and improve embryo cryotolerance.
Studies carried out by Matos et al.(79) investigated the effect of CLA in oocyte developmental competence after cryoprotectants exposure followed or not by vitrification and warming. They observed that CLA supplementation improved oocyte survival rates after vitrification, as well as improving cleavage rates, probably because of damage reduction. They also proposed that membrane permeability of both, water and cryoprotectants, would be influenced by the presence of CLA and, in this way, it was showed that bovine oocytes matured in a medium with CLA are more resistant to osmotic stress, reducing cellular cryodamage. Besides that, these oocytes presented a reduced rate of cryoprotectants influx (E.G. and DMSO 10%), which also can be responsible for the improvement of embryos quality. Taking together, these findings indicate that beyond the modulation of water and cryoprotectants flow through the cell membrane, CLA supplementation improves post-freezing oocyte viability, which provides a promising tool for female fertility preservation.
6. Effect of CLA supplementation on embryo cryopreservation
Lipid accumulation is associated with loss of embryonic viability and an increase in lesions caused by cryopreservation, especially in the early stages of embryonic development (80). Leite et al. (41). showed that the supplementation of culture media with trans-10 conjugated linoleic acid isomer, cis-12 reduced the deposition of intracytoplasmic lipids in embryonic cells. According to studies by Pereira et al. (6) the addition of CLA in the culture medium leads to a reduction in the expression of enzymes that participate in the synthesis of fatty acids, such as acylglycerol 3-phosphate acyltransferase responsible for catalyzing the synthesis of triglycerides, resulting, consequently, in reducing deposition lipid in embryonic cells.
in vitro produced (IVP) embryos are more sensitive to conventional freezing or vitrification than in vivo ones, making this one of the main obstacles to the expansion of cryopreservation technology (81, 82, 83). The reduced cryotolerance of IVP embryos, especially those cultured in a medium supplemented with fetal bovine serum (FBS), was correlated with an excessive accumulation of lipid droplets throughout embryonic development in vitro (84, 74, 4). Triacylglycerols correspond to most of the intracellular lipids found in oocytes and embryos (85, 69, 86) and, while in bovine embryos In vivo triacylglycerols represent 40-50% of the total lipid mass, in in vitro embryos they can reach 88% (86). In order to avoid the undesirable accumulation of lipids, several studies have attempted to replace FBS in the culture medium (87). Despite its detrimental effects on embryonic quality (88), FBS contains substances necessary for embryonic development, such as fatty acids, amino acids, vitamins, heavy metal chelators and growth factors (89) and, therefore, the difficulty of avoiding or finding a suitable substitute for FBS in the preparation of IVP media (42).
In this context, for embryo cryopreservation success, better techniques for cryopreservation must be developed or changes in the molecular composition of embryo culture media have to be done. The optimization of cryotechnics has shown limited success while changes in in vitro production systems have shown to be more promising, with the production of more cryotolerant and better-quality embryos (84). Emerging studies have found molecules, capable of modulating molecular mechanisms that inhibit the uptake of lipids by cells. One of the most promising molecules is CLA, which acts specifically on adipocytes, reducing the uptake of fatty acids without increasing lipolysis (90, 91). Supplementation of media containing serum for embryonic culture with CLA trans-10, cis-12 octadecadienoic (trans-10, cis-12 CLA) increased blastocyst cryosurvival rate within 24 hours of the post-heating culture (6). Bovine in vitro produced embryos in CLA containing medium, have also demonstrated more resistance to micromanipulation and cryopreservation. Moreover, the addition of trans-10, cis-12 CLA to the culture medium did not affect the cleavage rate, the sex ratio of the embryos, the quality or development of the blastocyst stage and significantly reduced lipid accumulation (6). In contrast, Dias et al. (92) evaluated the inclusion of CLA in the in vitro culture of bovine embryos and observed that it was not able to improve the embryonic response when using the slow freezing method.
More recently, Batista et al.(7) evaluated the effects of trans-10, cis-12 CLA on the development and cryotolerance of crossbred in vitro produced embryos. In those embryos cultured in CLA containing media, there was a reduction in the gene expression of the enzyme 1-acylglycerol 3-phosphate o-acyltransferase (AGPAT), a result that was associated with other findings, such as the reduction in the lipid content. However, a possible improvement in embryonic cryotolerance in response to CLA was not confirmed by hatching rates. These findings suggest that the reduction in the intracytoplasmic lipid accumulation caused by CLA, regardless of having a beneficial effect on reexpansion after cryopreservation, has not yet been sufficient to protect the embryo from harmful effects of cryopreservation (7).
7. Effects of CLA diet supplementation on fertility
Linoleic acid is a necessary nutrient for the growth and reproduction of non-ruminants and an important supplement that has a direct link between energy balance, postpartum and subsequent fertility (93, 94). Studies by Castañeda-Guitiérrez et al. (95) described that, in dairy cows, supplementation with trans-10 cis-12 CLA increased estradiol, androstenedione and IGF-I levels, important factors that support folliculogenesis. Likewise, Taylor et al.(96) showed better fertility performance 12 weeks prior to lactation and Darwash et al.(97) observed a strong correlation between the time of the first ovulation and the time of conception of dairy cows supplemented with CLA. One of the most well-known beneficial actions of CLA is to mitigate the postpartum negative energy balance (98, 99). Additionally, CLA also would be important to avoid premature birth, once activates metalloproteinases, inhibiting their functions which prevents premature rupture of fetal membranes and premature births (99). Rodney et al. (100) and Rodney et al. (101) in a meta-analysis observed that individual fats do not have the ability to increase fertility, but the investigation of the use of CLA has shown positive results, however, the number of studies is still insufficient. Veth et al. (99) evaluated studies that indicated a reduction in pregnancy time, from 151 to 117 days, when cows were supplemented with rumen-protected CLA. Abolghasemi et al. (102) suggest that the CLA-enriched diet has beneficial effects such as reduced expression of the receptor endocannabinoid (CNR2) and enzymes that synthesize fatty acid amides (NAPEPLD), in addition to an increase in PTGS2, resulting in an increase in plasma progesterone measurements during early lactation. Oliveira et al. (103) supplemented cows with CLA 18 days before parturition and observed that serum fat and β-hydroxybutyrate were reduced on days 1 and 7 postpartum, resulting in a lower prevalence of hyperketonemia on day 14 postpartum. Chandler et al. (104) observed that primiparous cows, when supplemented with CLA, showed a tendency to increase the conception rate in the first service, also leading to a shorter calving interval, corroborating studies carried out by Gutiérrez et al. (105). Csillik et al. (106) investigated the use of CLA in high production multiparous dairy cows and observed an increase in post-ovulatory P4, an increase in fertility with a reduction in the period of calving until conception, an increase in plasma levels of IGF-1 and leptin. The lipids present in the diet are crucial for the formation of the plasma membrane of the sperm (107), however, studies in males are very limited. Abdelatty et al. (58) suggest that supplementation with CLA in doses greater than 0.5% for long term in male rabbits is not beneficial, despite the beneficial effects on growth, it does not balance the negative effects of fertility. Zamora-Zamora et al. (108) evaluated the inclusion of CLA in the diet at 1% of wild boars and did not observe differences regarding semen characteristics and fatty acid profile of sperm. Overall, diet supplementation with CLA would be an important strategy to enhance the reproductive performance of domestic mammals and is a field that requires further investigations.
Conclusion
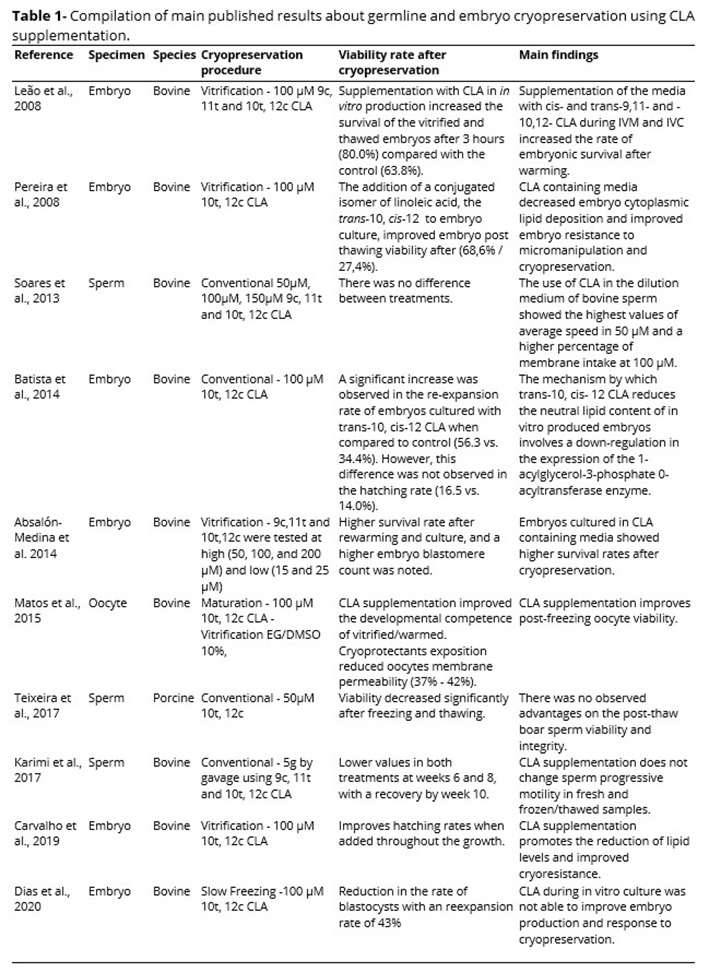
The function of CLA in cryopreservation is exercised by two mechanisms: i) modulation of the lipid profile of the membrane and ii) amount of intracellular lipids. The effects of CLA supplementation on sperm, oocyte, and embryo cryotolerance are briefly compiled in Table 1. In sperm cryopreservation, the evidence suggests that CLA modulates sperm function mainly by modulating the lipid profile in the membrane. In sperm cryopreservation, the evidence suggests that CLA modulates exerts its effect on cryopreservation especially by modulating the lipid profile in the membrane. However, these effects on sperm cryopreservation are minimal or even negative, especially when supplemented in the diet. In oocytes and embryos, the evidence suggests that CLA acts both at the level of the lipid profile in the membrane and in the amount of intracellular lipids. In these cells, the information in the literature demonstrates a beneficial effect of these fatty acids on cryopreservation. CLA supplementation in oocyte, during in vitro maturation or cryopreservation, has improved both viability after freezing and their developmental competence, representing a very promising strategy to produce more cryotolerant embryos. Otherwise, the decrease of intracytoplasmic lipid content observed in embryos cultured in CLA containing media, and its positive effect on embryo survival after cryopreservation open new avenues in embryo production and cryobiology. Finally, further studies are necessary in order to evaluate CLA effects on gonad, oocyte, sperm, and embryo cryopreservation.
Author contribution
All the authors contributed to the writing of the manuscript, and all authors approve the final version.
Declaration of Competing Interest
The authors declare that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Acknowledgments
This work was supported by the National Council for Scientific and Technological Development (CNPq, Brazil) and Minas Gerais State Research Foundation (FAPEMIG, Brazil). DSF, GAGL, BRN and LAACP received a scholarship from CNPq, CAPES or FAPEMIG.
References
1. Jang TH, Park SC, Yang JH, Kim JY, Seok JH, Park US, Choi CW, Lee SR, Han, J. Cryopreservation and its clinical applications. Integr Med Res Epub 2017;1:12-18. Disponível em: 10.1016/j.imr.2016.12.001
2. Pegg DE. Principles of Cryopreservation. In: Wolkers W., Oldenhof H. (eds) Cryopreservation and Freeze-Drying Protocols. Methods in Molecular Biology (Methods and Protocols), 2015;3-19. Disponível em: https://www.springer.com/gp/book/9781493921928
3. Doyong G, Critser JK. Mechanisms of Cryoinjury in Living Cells ILAR Journal, 2000; 41(4): 187-196. Disponível em: 10.1093/ilar.41.4.187
4. Lapa M, Marques CC, Alves SP, Vasques MI, Baptista MC, Carvalhais I. Effect of trans-10 cis-12 conjugated linoleic acid on bovine oocyte competence and fatty acid composition. Reprod Domest Anim, 2011;46: 904-910. Disponível em: 10.1111/j.1439-0531.2011.01762.x
5. Teixeira SMP, Chaveiro AEN, Silva JFM. Effect of trans-10, cis-12 isomer of conjugated linoleic acid on boar smen quality after cryopreservation. Animal Reproduction, 2017;14(2):400-405. Disponível em: 10.21451/1984-3143-AR831
6. Pereira, R.M.; Baptista, M.C.; Vasques, M.I.; Horta, A.E.; Portugal, P.V.; Bessa, R.J.; 2007. Cryosurvival of bovine blastocysts is enhanced by culture with trans-10 cis-12 conjugated linoleic acid. Anim Reprod Sci, 2007; 98: 293-301. Disponível em: DOI: 10.1016/j.anireprosci.2006.03.015
7. Batista RITP, Raposo NRB, Campos-Junior PHA, Pereira MM, Camargo LSA, Carvalho BC, Gama MAS, Viana JHM. Trans-10, cis-12 conjugated linoleic acid reduces neutral lipid content and may affect cryotolerance of in vitro- produced crossbred bovine embryos. Journal of Animal Science and Biotechnology, 2014;5:33. Disponível em: 10.1186/2049-1891-5-33
8. Bessa RJB, Santos-Silva J, Ribeiro JMR. Reticulo-rumen biohydrogenation and the enrichment of ruminant edible products with linoleic acid conjugated isomers. Livestock Production Science, 2000; 63: 201-211. Disponível em: https://doi.org/10.1016/S0301-6226(99)00117-7
9. Pariza MW, Ashoor SH, Chu FS, Lund DB. Effect of temperature and time on mutagen formation in pan-fried hamburger. Cancer Letters, Amsterdam, 1979; 7: 63-69. Disponível em: DOI: 10.1016/s0304-3835(79)80097-x
10. Park Y, Albright KJ, Liu W, Storkson JM, Cook ME, Pariza MW. Effect of conjugated linoleic acid on body composition in mice. Lipids, 1997; 32: 853–858. Disponível em: DOI: 10.1007/s11745-997-0109-x
11. Dugan MER, Aalhus JL, Jeremiah LE, Kramer JKG, Schaefer AL. The effects of feeding conjugate linoleic acid on subsequent pork quality. Canadian Journal of Animal Science, 1999; 79: 45-51. Disponível em: https://doi.org/10.4141/A98-070
12. Bee G. Dietary conjugated linoleic acids clatter adipose tissue and milk lipids of pregnant and lactating sows. 1997. Journal of Nutrition, 2000; 130: 2292-2298.
13. Baumgard LH, Corl BA, Dwyer DA, Saebo A, Bauman DE. Identification of the conjugated linoleic acid isomer that inhibits fat synthesis. American Journal Physiology Regulatory Comparative Physiology, 2000; 278: 179-84. Disponível em: DOI: 10.1152/ajpregu.2000.278.1.R179.
14. Sebedio JL, Gnaedig S, Chardigny JM. Recent advances in conjugated linoleic acid research. Current Opinion Clinical Nutrition Metabolic Care,1999; 2(6): 499-506.
15. Bauman DE, Griinari JM. Regulation and nutritional manipulation of milk fat: low-fat milk syndrome. Livestock Production Science,2001; 70: 15-29. Disponível em: https://doi.org/10.1016/S0301-6226(01)00195-6
16. Kepler CR, Hirons KP, McNeill JJ, Tove SB. Intermediates and products of the biohydrogenation of linoleic acid by Butyrivibrio fibrisolvens. Journal of Biological Chemistry, 1966; 241: 1350-1354.
17. Harfoot CG, Halzlewood GP. Lipid metabolism in the rumem. Hobson, P.N. (Ed.). The rumen microbial ecosystem, New: Elsevier, 1988: 527.
18. Griinari JM, Bauman DE. Biosynthesis of CLA and incorporation into milk fat. Yurawecz, M. P. et al., Advances in Conjugated Linoleic Acid Research, AOCS Press, Champaign, IL,1999: 180-200. Disponível em: https://doi.org/10.1093/jn/130.9.2285
19. Coakley M, Ross RP, Nordgren M, Fitzgerald G, Dever R, Stanton C. Conjugated linoleic acid biosynthesis by human derived Bifidobacterium species. Journal of Applied Mocrobiology, 2003; 94: 138-145. Disponível em: DOI: 10.1046/j.1365-2672.2003.01814.x
20. Ando A, Ogawa J, Kishino S, Shimizu S. Conjugated linoleic acid production from castor oil by Lactobacillus plantarum JCM 1551. Microbial and Enzyme Technology, 2004 35: 40-45. Disponível em: DOI: 10.1016/j.enzmictec.2004.03.013
21. Stewart CS, Flynt HJ, Bryant MP. The rumen bacteria. in The Rumen Microbial Ecosystem. P. N. Hobson and C. S. Stewart, ed. Blackie Academic and Professional, New York, NY, 1997; 10-72.
22. Griinari JM, Dwyer DA, MCGuire MA, Bauman DE, Palmiquist DL, Nurmela KVV. Trans-octadecenoic acid and milk fat depression in lactating dairy cows. Journal of Dairy Science, 1998; 81: 1251-1261. Disponível em: https://doi.org/10.3168/jds.S0022-0302(98)75686-3
23. Chouinard PY, Bauman DE, Corl BA, Baumard LH, McGuire MA, Giesy JG. An update on conjugated linoleic acid. Cornell Nutrition Conference Feed Manufacturers, 1999; 93-101.
24. Griinari JM, Corl BA, Lacy SH, Chouinard PY, Nurmela KV, Bauman DE. Conjugated linoleic acid is synthesized endogenously in lacting dairy cows by Δ9 desaturase. Journal Nutrition, 2000; 130(9): 2285-2291. Disponível em: DOI: 10.1093/jn/130.9.2285
25. Perfield JW, Bernal-Santos G, Overton TR, Bauman DE. Effects of dietary supplementation of rumen protected conjugated linoleic acid dairy cows during established lactation. Journal of Dairy Science, 2002; 85: 2609-2617. Disponível em: https://doi.org/10.3168/jds.S0022-0302(02)74346-4
26. Corl BA, Baumgard LH, Dwyer DA, Griinari JM, Philips BS, Bauman DE. The role of delta-9-desaturase in the production of cis-9, trans-11. Journal of Nutritional Biochemistry,2001; 12: 622-630. Disponível em: DOI: http://doi.org/10.1016/s0955-2863(01)00180-2
27. Kay JK, Mackle TR, Auldist MJ, Thomson NA, Bauman DE. Endogenous synthesis and enhancement of conjugated linoleic acid in pasture-fed dairy cows. Proceedings of New Zealand Society Animal Production,2002; 62: 12-15.
28. Lee KN, Pariza MW, Ntambi JM. Conjugated linoleic acid decreases hepatic stearoyl-CoA desaturase mRNA expression. Biochemical and Biophysical Research Communications, 1998; 248: 817-821. Disponível em: https://doi.org/10.1006/bbrc.1998.8994
29. Park Y, Storkson JM, Ntambi JM, Cook ME, Sih CJ, Pariza MW. Inhibition of hepatic stearoyl-CoA desaturase activity by trans-10, cis-12 conjugated linoleic acid and its derivatives. Biochemical et Biophysical Acta,2000; 1486: 285-292. Disponível em: DOI: http://doi.org/10.1016/s1388-1981(00)00074-3
30. Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. Journal Clinic Invest, 2002; 109: 1125–1131. Disponível em: doi: http://doi.org/10.1172/JCI15593
31. Baumgard LH, Matitashvili E, Corl BA, Dwyer DA, Bauman DE. Trans-10, cis-12 conjugated linoleic acid decreases lipogenic rates and expression of genes involved in milk lipid synthesis in dairy cows. Journal of Dairy Science, 2002; 85: 2155. Disponível em: https://doi.org/10.3168/jds.S0022-0302(02)74294-X
32. Peterson DG Matitashvili EA, Bauman DE. Diet-induced milk fat depression in dairy cows results in increased trans-10, cis-12 CLA in milk fat and coordinate suppression of mRNA abundance for mammary enzymes involved in milk fat synthesis. J Nutr, 2003; 133: 3098-3102. Disponível em: DOI: http://doi.org/10.1093/jn/133.10.3098
33. Granlund L, Pedersen JI, Nebb HI. Impaired lipid accumulation by trans-10 cis-12 during adipocyte differentiation is dependent on timing and length of treatment. Biochim Biophys Acta, 2005; 1687:11-22. Disponível em: DOI: http://doi.org/10.1016/j.bbalip.2004.08.018
34. Pereira RM, Marques CC. Animal oocyte and embryo cryopreservation. Cell Tissue Bank, 2008; 9:267. Disponível em: DOI: http://doi.org/10.1007/s10561-008-9075-2
35. Al Darwich A, Perreau C, Petit MH, Papillier P, Dupont J, Guillaume D. Effect of PUFA on embryo cryoresistance, gene expression and AMPKα phosphorylation in IVF-derived bovine embryos. Prostaglandins Other Lipid Mediat, 2011; 93: 30-36. Disponível em: DOI: http://doi.org/10.1016/j.prostaglandins.2010.06.002
36. Singer SJ, Nicolson GL. The fluid mosaic model of the structure of cell membranes. Science, 1972; 18;175(4023):720-31. Disponível em: DOI: http://doi.org/10.1126/science.175.4023.720
37. Watson H. Biological membranes. Essays Biochem, 2015; 15(59): 43-69. Disponível em: doi: http://doi.org/10.1042/bse0590043
38. Vajta G, Nagy ZP. Are programmable freezers still needed in the embryo laboratory? Review on vitrification. Reprod Biomed Online, 2006; 12: 779-796. Disponível em: DOI: http://doi.org/10.1016/s1472-6483(10)61091-7
39. Juchem SO, Cerri RLA, Villaseñor M, Galvão KN, Bruno RGS, Rutigliano HM, Depeters EJ, Silvestre FT, Thatcher WW, Santos JEP. Supplementation with calcium salts of linoleic and trans-octadecenoic acids improves fertility of lactating dairy cows. Reprod Dom Anim, 2010;45: 55–62. Disponível em: https://doi.org/10.3168/jds.2008-1614
40. Rahme LSTR. Efeito do ácido linoléico conjugado na sobrevivência pós criopreservação de embriões bovinos produzidos in vitro. Dissertação (Mestrado em Ciência Animal). Escola de Veterinária, Universidade Federal de Minas Gerais, MG, 2012. In: http://hdl.handle.net/1843/BUOS-8UHG53
41. Leite AC, Andrade VB, Silva EBM, Borges AM. Effect of conjugated linoleic acid addition in in vitro culture medium in F1 Holstein X Zebu embryo survival post vitrification. Arq. Bras. Med. Vet. Zootec, 2017; 69 (6): 1385-1392. Disponível em: http://dx.doi.org/10.1590/1678-4162-9238.
42. Leão BCS, Rocha-Frigoni NAS, Cabral EC, Coelho MB, Ferreira CR, Eberlin MN, Accorsi MF, Nogueira E, Mingoti GZ. Improved embryonic cryosurvival observed after in vitro supplementation with conjugated linoleic acid is related to changes in the membrane lipid profile. Theriogenology, 2015; 84: 127-136. Disponível em: https://doi.org/10.1016/j.theriogenology.2015.02.023
43. Carvalho BP, Queirós Costa FQ, Detoni D, Rosa FB, Dias AJB. Use of conjugated linoleic acid (trans-10, cis-12) to cultivate bovine embryos: effect on cryoresistance and lipid content. R. Bras.Zootec., 2019; 48. Disponível em: http://dx.doi.org/10.1590/rbz4820180322
44. Hess RA, França LR. 2007 Spermatogenesis and the cycle of the seminiferous epithelium. In Molecular Mechanisms in Spermatogenesis (ed. Cheng, C.Y.), 2007; 1–15. Disponível em: DOI: http://doi.org/10.1007/978-0-387-09597-4_1
45. Siqueira JB, Guimarães JD, Costa EP, Henry M, Torres CAA, Silva MVGB, Silveira TS. Relação da Fertilidade de Sêmen bovino congelado com testes de avaliação espermática. Revista Brasileira de Zootecnia / Brazilian Journal of Animal Science, 2007; 36: 387-395
46. Bedford JM, Hoskins DD. The mammalian spermatozoon: morphology, biochemistry and physiology. In: Lamming, G. E. Marshall's Physiology of Reproduction, London, Churchill Livingstone, 1990;2: 379-568.
47. Albert DH, Coniglio JG. Metabolism of eicosa-11,14-dienoic acid in rat testes. Evidence for delta8-desaturase activity. Bioch Biophys Acta, 1977; 21;489(3):390-6.
48. Ladha S. Lipid heterogeneity and membrane fluidity in a highly polarized cell, the mammalian spermatozoon. Journal of Membrane Biology, 1998;165: 1–10. Disponível em: https://doi.org/10.1007/s002329900415
49. White I. Lipids and calcium uptake of sperm in relation to cold shock and preservation: A review. Reproduction, Fertility and Development, 1993; 5: 639–658. Disponível em: DOI: http://doi.org/10.1071/rd9930639
50. Am-In N, Kirkwood R, Techakumphu M, Tantasuparuk W. Lipid profiles of sperm and seminal plasma from boars having normal or low sperm motility. Theriogenology, 2011; 75: 897–903. Disponível em: http://doi.org/10.1016/j.theriogenology.2010.10.032
51. Conquer JA, Martin JB, Tummon I, Watson L, Tekpetey F. Effect of DHA supplementation on DHA status and sperm motility in asthenozoospermic males. Lipids, 2000; 35: 149–154. Disponível em: DOI: http://doi.org/10.1007/BF02664764
52. Samadian F, Towhidi A, Rezayazdi K, Bahreini M. Effects of dietary n-3 fatty acids on characteristics and lipid composition of ovine sperm. Animal, 2010; 4: 2017–2022. Disponível em: https://doi.org/10.1017/S1751731110001308
53. Gholami H, Chamani M, Towhidi A, Fazeli MH. Effect of feeding a docosahexaenoic acid-enriched nutriceutical on the quality of fresh and frozen-thawed semen in Holstein bulls. Theriogenology, 2010; 74: 1548-1558. Disponível em: DOI: http://doi.org/10.1016/j.theriogenology.2010.06.025
54. Moallem U, Neta N, Zeron Y, ZAchut M, Roth Z. Dietary α-linolenic acid from flaxseed oil or eicosapentaenoic and docosahexaenoic acids from fish oil differentially alter fatty acid composition and characteristics of fresh and frozen-thawed bull semen. Theriogenology, 2015; 83: 1110-1120. Disponível em: DOI: http://doi.org/10.1016/j.theriogenology.2014.12.008
55. Surai P, Noble R, Sparks N, Speake B. Effect of long-term supplementation with arachidonic or docosahexaenoic acids on sperm production in the broiler chicken. Journal of Reproduction and Fertility, 2000; 120: 257–264.
56. Rooke JA, Shao CC, Speake BK. Effects of feeding tuna oil on the lipid composition of pig spermatozoa and in vitro characteristics of semen. Reproduction, 2001; 121: 315–322. Disponível em: DOI: http://doi.org/10.1530/rep.0.1210315
57. Stulnig TM, Huber J, Leitinger N, Imre EM, Angelisová P, Nowotny P, Waldhäusl W. Polyunsaturated eicosapentaenoic acid displaces proteins from membrane rafts by altering raft lipid composition. Journal of Biological Chemistry, 2001; 276: 37335–37340. Disponível em: doi: http://doi.org/10.1074/jbc.M106193200
58. Abdelatty AM, Badr OAM, Mohamed SA, Khattab MS, Dessouki SM, Farid OAA, Elolimy AA, Sakr OG, Elhady MA, Mehesen G, Bionaz M. Long term conjugated linoleic acid supplementation modestly improved growth performance but induced testicular tissue apoptosis and reduced sperm quality in male rabbit. PLoS ONE, 2020; 15(1). Disponível em: DOI: http://doi.org/10.1371/journal.pone.0231280
59. Karimi R, Towhidi A, Zeinoaldini S, Rezayazdi K, Mousavi M, Safari H, Martinez-Pastor F. Effects of supplemental conjugated linoleic acids (CLA) on fresh and post-thaw sperm quality of Holstein bulls. Reprod Dom Anim., 2017; 1-9. Disponível em: DOI: http://doi.org/10.1111/rda.12932
60. Maldjiana A, Pizzi F, Gliozzic T, Cerolinibi S, Penny P, Noble R. Changes in sperm quality and lipid composition during cryopreservation of boar sêmen. Theriogenology, 2005; 63: 411-421. Disponível em: DOI: http://doi.org/10.1016/j.theriogenology.2004.09.021
61. Vasquez JM, Roldan ERS. Phospholipid metabolism in boar spermatozoa and role of diacylglirecol species in the novo formation of phosphatidylcholine. Mol Rep, 1997; 47: 105-112. Disponível em: DOI: http://doi.org/10.1002/(SICI)1098-2795(199705)47:1<105::AID-MRD14>3.0.CO;2-0
62. Pérez-Pé R, Cebrian-Pérez JA, Muiño-Blanco T. Semen plasma protein prevent cold-shock membrane damage to ram spermatozoa, Theriogenology, 2001; 56: 425–434. Disponível em: https://doi.org/10.1016/S0093-691X(01)00574-X
63. Hossain M.D.S, Tareq K, Hammano H, Tsujii KMA. Effect of fatty acids o boar sperm motility, viability and acrosome reaction, Reprod. Med. Biol., 2007; 6: 235–239. Disponível em: doi: http://doi.org/10.1111/j.1447-0578.2007.00191.x
64. Takahashi T, R. Itoh H. Nishinomiya M, Katoh N. Manabe, Effect of linoleic acid albumin in a dilution solution and long-term equilibration for freezing of bovine spermatozoa with poor freezability, Reprod. Domest. Anim, 2012; 47: 92–97. Disponível em: https://doi.org/10.1111/j.1439-0531.2011.01806.x
65. Kaeoket K. Cryopreservation of boar spermatozoa: an important role of antioxidants. Katkov I (Ed.). Current Frontiers in Cryopreservation. Rijeka, Croatia: InTech, 2012; 139-164.
66. Soares MP, Brandelli A, Celeghini ECC, Arruda RP, Rodriguez SAF. Effect of cis-9, trans-11 and trans-10, cis-12 isomers of conjugated linoleic acid on the integrity and functionality of cryopreserved bovine spermatozoa. Cryobiology, 2013; 67: 102-105. Disponível em: DOI: 10.1016/j.cryobiol.2013.05.003
67. Verstegan J, Iguer-Quada M, Onclin K. Computer assisted semen analyzers in andrology research and veterinary practice. Theriogenology, 2002; 57: 149-179. Disponível em: http://dx.doi.org/10.1016 / S0093-691X (01) 00664-1.
68. Hummitzsch K, Irving-Rodgers HF, Hatzirodos N, Bonner W, Sabatier, Reinhardt DP, Sado Y, Ninomiya Y, Wilhelm D, Rodgers RJ. A new model of development of the ovary and mammalian follicles. Plos One, 2013; 8. Disponível em: DOI: http://doi.org/10.1371/journal.pone.0055578
69. Ferguson EM, Leese HJ. Triglyceride content of bovine oocytes and early embryos. J. Reprod Fert, 1999; 116: 373-378. Disponível em: DOI: http://doi.org/10.1530/jrf.0.1160373
70. Donnez J, Dolmans MM. Fertility preservation on women. N Engl J Med, 2017; 377: 1657–1665. Disponível em: DOI: http://doi.org/10.1056/NEJMra1614676
71. De Santis L, Coticchio G, Paynter S, Albertini D, Hutt K, Cino I. Permeability of human oocytes to ethylene glycol and their survival and spindle configurations after slow cooling cryopreservation. Hum Reprod., 2007; 22: 2776–2783. Disponível em: https://doi.org/10.1093/humrep/dem240
72. Gualtieri R, Iaccarino M, Mollo V, Prisco M, Iaccarino S, Talevi R. Slow cooling of human oocytes: ultrastructural injuries and apoptotic status. Fertil Steril, 2009; 91: 1023–34. Disponível em: DOI:https://doi.org/10.1016/j.fertnstert.2008.01.076
73. Arav A. Cryopreservation of oocytes and embryos. Theriogenology, 2017; 81 :96–102. Disponível em: DOI: http://doi.org/10.1016/j.theriogenology.2013.09.011
74. Pereira RM, Marques CC, Baptista MC, Vasques MI, Horta AEM. Influência da suplementação de ácido araquidônico e inibição da ciclooxigenase / lipoxigenase no desenvolvimento de embriões bovinos precoces. Rev Bras. Zoot, 2006; 35: 1-6.
75. Isachenko V, Isachenko E, Michelmann HW, Alabart JL, Vazquez I, Bezugly N, Nawroth F. Lipolysis and ultrastructural changes of intracellular lipid vesicles after cooling of bovine and porcine GV-oocytes. Anat Histol Embryol, 2001; 30:333–338. Disponível em: DOI: http://doi.org/10.1046/j.1439-0264.2001.00339.x
76. Silva-Santos, K. C., Ferreira, C. R., Santos, G. M. G., Eberlin, M. N., Siloto, L. S., Rosa, C. O., Marcantonio, T. N., and Seneda, M. M. MALDI-MS lipid profiles of oocytes recovered by ovum pickup from Bos indicus and 1/2 indicus_taurus with high vs low oocyte yields. Reprod. Domest. Anim. 2014, 49, 711–718. Disponível em: doi: http://doi.org/10.1111/rda.12352
77. Aardema, H., Vos, P. L. A. M., Lolicato, F., Roelen, B. A. J., Knijn, H. M., Vaandrager, A. B., Helms, J. B., and Gadella, B. M. Oleic acid prevents detrimental effects of saturated fatty acids on bovine oocyte developmental competence. Biol. Reprod. 2011, 85, 62–69. Disponível em: https://doi.org/10.1095/biolreprod.110.088815
78. Christina R, Ferreira AF, Alan K, Jarmusch A, Valentina Pirro A, Clint M, AlfaroA, Andres F, Gonzalez-Serrano BE, Heiner Niemann B, Matthew B, Wheeler C, Rathnaweera AC, Rabel C, Judy E, Hallett D, Rebecca Houser D, Annemarie Kaufman D, Graham Cooks RA. Ambient ionisation mass spectrometry for lipid profiling and structural analysis of mammalian oocytes, preimplantation embryos and stem cells. Reproduction, Fertility and Development, 2015, 27, 621–637. Disponível em: http://dx.doi.org/10.1071/RD14310.
79. Matos JE, Marques CC, Moura TF, Baptista MC, Horta AEM, Soveral G, Pereira RMLN. Conjugated linoleic acid improves oocyte cryosurvival through modulation of the cryoprotectants influx rate. Biology and Endocrinology, 2015; 13:60. Disponível em: https://doi.org/10.1186/s12958-015-0059-3
80. Almiñana C, Cuello C. What is new in the cryopreservation of embryos? Anim. Reprod., 2015; 12(3): 418-427.
81. Saragusty J, Arav A. Current progress in oocyte and embryo cryopreservation by slow freezing and vitrification. Reproduction, 2011; 141: 1-19. Disponível em: DOI: http://doi.org/10.1530/REP-10-0236
82. Pollard JW, Leibo SP. Comparative cryobiology of in vitro and In vivo derived bovine embryos. Theriogenology, 1993; 39: 287.
83. Hasler JF, Henderson WB, Hurtgen PJ, Zin ZK, McCauley AD, Mower SA, Neely B, Shuey LS, Stokes JE, Trimmer SA. Production freezing and transfer of bovine IVF embryos and subsequent calving results. Theriogenology, 1995; 42: 141-152.
84. Seidel Jr GE. Modifying oocytes and embryos to improve their cryopreservation. Theriogenology, 2006; 65: 228-235. Disponível em: DOI: http://doi.org/10.1016/j.theriogenology.2005.09.025
85. McEvoy TG, Coull GD, Broadbent PJ, Hutchinson JS, Speak BK. Fatty acid composition of lipids in immature cattle, pig and sheep oocytes with intact zona pellucida. J Reprod Fertil, 2000; 118: 163-170.
86. Charpigny G, Guesnet P, Marquant-LeGuiene B, Heyman Y, Mermillod P, Humblot P. Fatty acid composition of triglycerides, phosphatidylcholines and phosphatidylethanolamines of bovine embryos. Les actes du BRG, 2003; 4: 159-172.
87. Mingoti GZ, Caiado-Castro VS, Méo SC, Barretto LS, Garcia JM. The effect of interaction between macromolecule supplement and oxygen tension on bovine oocytes and embryos cultured in vitro. Zygote, 2009; 17: 321-328. Disponível em: DOI: http://doi.org/10.1017/s0967199409005450
88. Rizos D, Gutiérrez-Adán A, Pérez-Garnelo S, de la Fuente J, Boland MP, Lonergan P. Bovine embryo culture in the presence or absence of serum: implications for blastocyst development, cryotolerance, and messenger RNA expression. Biol Reprod, 2003; 68: 236-243. Disponível em: https://doi.org/10.1095/biolreprod.102.007799
89. Stein A. Decreasing variability in your cell culture. Biotechniques, 2007; 43: 228-229. Disponível em: DOI: http://doi.org/10.2144/000112561
90. Pariza MW, Park Y, Cook ME. The biologically active isomers of conjugated linoleic acid. Progress in Lipid Research, 2001; 40: 283-298. Disponível em: http://doi.org/10.1016/s0163-7827(01)00008-x
91. Sehat N, Kramer JK, Mossoba MM, Yurawecz MP, Roach JA, Eulitz K. Identification of conjugated linoleic acid isomers in cheese by gas chromatography, silver ion high performance liquid chromatography and mass spectral reconstructed ion profiles. Comparison of chromatographic elution sequences Lipids, 1998; 33: 963-971. Disponível em: DOI: http://doi.org/10.1007/s11745-998-0293-8
92. Dias, R.L.O.; Leme, L.O.; Sprígigo, J.F.W.; Pivato, I; Dode, M.A.N. 2020. Effect of delipidant agents during in vitro culture on the development, lipid content, gene expression and cryotolerance of bovine embryos. Reprod Dom Anim., 2020; 55:11–20. Disponível em: https://doi.org/10.1111/rda.13579
93. NRC. Nutrient Requirements of laboratory Animals. 4h rev. ed. Natl. Acad. Sci., Washington, DC, 1995. Disponível em: DOI: http://doi.org/10.17226/4758
94. Butler WR. Nutritional interactions with reproductive performance in dairy cattle. Anim. Reprod. Sci., 2000; 60-61: 449-457. Disponível em: DOI: http://doi.org/10.1016/s0378-4320(00)00076-2
95. Castañeda-Guitiérrez E, Benefield BC, Veth MJ, Santos NR, Gilbert RO, Butler WR, Bauman DE. 2007 Evaluation of the mecanism of action of conjugated linoleic acid isomers on reproduction of dairy cows. Journal of Dairy Science, 2007; 90: 4253 - 4264. Disponível em: DOI:https://doi.org/10.3168/jds.2007-0117
96. Taylor CG, Zahradka P. Dietary conjugated linoleic acid and insulin sensitivity and resistance in rodent models. The American Journal of Clinical Nutrition, 2004; 79(6): 1164S–1168S. Disponível em: DOI: http://doi.org/10.1093/ajcn/79.6.1164s
97. Darwash AO, Lamming GE, Woolliams JA. The phenotypic association between the interval to post-partum ovulation and traditional measures of fertility in dairy cattle Anim. Sci., 1997; 65: 9-16. Disponível em: DOI: https://doi.org/10.1017/S1357729800016234
98. Griinari JM, Bauman DE. Milk fat depression: concepts, mechanisms and management applications. In: Sejrsen, K.; Hvelplund, T.; Nielsen, M. O. Ruminant physiology: digestion, metabolism and impact of nutrition on gene expression, immunology and stress. Wageningen, The Netherlands: Wageningen Academic, 2006; 389–417.
99. Veth MJ, Bauman DE, Koch W, Mann GE, Pfeiffer AM, Butler WR. Efficacy of conjugated linoleic acid for improving reproduction: A multi-study analysis in early-lactation dairy cows. Journal of Dairy Science, 2009; 92: 2662–2669. Disponível em: DOI:https://doi.org/10.3168/jds.2008-1845
100. Rodney RM, Celi P, Scott W, Breinhild K, Lean IJ.Effects of dietary fat on fertility of dairy cattle: A meta-analysis and meta-regression. Dairy Sci. 98:1–20 © American Dairy Science Association®, 2015. Disponível em: http://dx.doi.org/10.3168/jds.2015-9528
101. Rodney RM, Celi P, Scott W, Breinhild K, Lean IJ.Effects of nutrition on the fertility of lactating dairy cattle Author links open overlay panel. Journal of Dairy Science Volume 101, Issue 6, June 2018, Pages 5115-5133 Disponível em: https://doi.org/10.3168/jds.2017-14064
102. Abolghasemi A, Dirandeh E, Ansari pirsaraei Z, Shohreh B. Dietary conjugated linoleic acid (CLA) supplementation alters the expression of genes involved in the endocannabinoid system in the bovine endometrium and increases plasma progesterone concentrations, Theriogenology (2016), Disponível em: doi: http://doi.org/10.1016/j.theriogenology.2016.05.003.
103. Oliveira RC, Pralle RS, de Resende LC, Nova CHPC, Caprarulo V, Jendza JA, et al. (2018) Prepartum supplementation of conjugated linoleic acids (CLA) increased milk energy output and decreased serum fatty acids and β-hydroxybutyrate in early lactation dairy cows. PLoS ONE 13(5): e0197733. Disponível em: https://doi.org/10.1371/journal. pone.0197733
104. Tawny L, Chandler A, Robert T, Fugatea, Joshua A, Jendza B, Arnulf Troescher C, Heather M. White A. Conjugated linoleic acid supplementation during the transition period increased milk production in primiparous and multiparous dairy cows. Animal Feed Science and Technology 224 (2017) 90–103. Disponível em: http://dx.doi.org/10.1016/j.anifeedsci.2016.12.008.
105. Castaneda-Gutiérrez E, Overton TR, Butler WR, Bauman DE. Dietary supplements of two doses of calcium salts of conjugated linoleic acid during the transition period and early lactation. J. Dairy Sci. 2005. 88, 1078–1089. Disponível em: DOI: http://doi.org/10.3168/jds.S0022-0302(05)72775-2
106. Z. Csillik,*1 V. Faigl,† M. Keresztes,† E. Galamb,‡ H. M. Hammon,§ A. Tröscher,* H. Fébel,# M. Kulcsár,† F. Husvéth,‡ Gy. Huszenicza,† and W. R. Butler‖. Effect of pre- and postpartum supplementation with lipid-encapsulated conjugated linoleic acid on reproductive performance and the growth hormone–insulin-like growth factor-I axis in multiparous high-producing dairy cows. J. Dairy Sci. 100:1–11 Disponível em: https://doi.org/10.3168/jds.2016-12124.
107. Gliozzi TM, Zaniboni L, Maldjian A, Luzi F, Maertens L, and Cerolini S. Quality and lipid composition of spermatozoa in rabbits fed DHA and vitamin E rich diets. Theriogenology. 2009; 71(6), pp. 910–919. Disponível em: https://doi.org/10.1016/j.theriogenology.2008.10.022
108. Zamora-Zamora V, Figueroa-Velasco JL, Cordero-Mora JL, Nieto-Aquino R, García-Contreras ADC, Sánchez-Torres MT. Conjugated linoleic acid supplementation does not improve boar semen quality and does not change its fatty acid profile. Veterinaria México. 2017; 4(3), pp. 1–15. Disponível em: doi: http://doi.org/10.21753/vmoa.4.3.387.
109. Absalón-Medina VA, Bedford-Guaus SJ, Gilbert RO, Siqueira LC, Eposito G, Schneider A, Cheong SH, Butler WR.The effects of conjugated linoleic acid isomers cis-9,trans-11 and trans-10,cis-12 on in vitro bovine embryo production and cryopreservation. Journal of Dairy Science Volume 97, Issue 10, October 2014, Pages 6164-6176. Disponível em: DOI: http://doi.org/10.3168/jds.2013-7719