

Abstract
Whilst considering the need anesthetic techniques supported by species-specific morphology, it has been sought to describe the morphometry of the Hoary Fox's infraorbital foramen with aims at correlating its topography with points of anatomic reference in the skull, thereby providing subsidy for a more effective local anesthetic block in that species. Four skulls of Lycalopex vetulus had been used, from which all of the measurements in each antimere were performed. The infraorbital foramen was located at the maxilla bone, dorsally-wise from the third upper pre-molar tooth, and, from the ventral end of its caudal margin, it would stand apart from the alveolar margin of that bone by 4.19 mm, in average; from the orbital margin at the level of the lacrimal foramen by 14.10 mm; from the dorsal end of the frontal process of the zygomatic bone by 37.10 mm; from the rostral end of the alveolar margin of the first upper incisor tooth by 38.54 mm; and, from the caudal end of the nuchal crest by 100.53mm - at the level of the median sagittal plane; as it also presented a sagittal axis of 5.21 mm in average. It is suggested that, for the Hoary Fox, the needle be introduced by 4.19 mm in contact with the maxilla bone, in a perpendicular fashion, and in a ventral-dorsal orientation from the alveolar margin of the same - whilst using, as an anatomic reference, the diastema that exists between the third and the fourth upper pre-molar teeth.
Keywords: anesthesiology; applied morphology; veterinary dentistry
Resumo
Considerando-se a necessidade de técnicas anestésicas respaldadas pela morfologia espécie-específica, objetivou-se descrever a morfometria do forame infraorbital de Raposa-do-campo a fim de correlacionar sua topografia com pontos de referência anatômica no crânio, oferecendo subsídio para um bloqueio anestésico local mais efetivo nesta espécie. Foram utilizados quatro crânios de Lycalopex vetulus, a partir dos quais foram realizadas todas as mensurações em cada antímero. O forame infraorbital localizou-se no osso maxila, dorsalmente ao terceiro dente pré-molar superior e, a partir do extremo caudal de sua margem ventral, distanciou-se, em média, 4,19 mm da margem alveolar desse osso; 14,10 mm da margem orbital ao nível do forame lacrimal; 37,10 mm do extremo dorsal do processo frontal do osso zigomático; 38,54 mm do extremo rostral da margem alveolar do dente incisivo medial superior; e 100,53 mm do extremo caudal da crista nucal ao nível do plano sagital mediano; além de apresentar um eixo sagital com uma média de 5,21 mm. Para a Raposa-do-campo sugere-se que a agulha seja introduzida por 4,19 mm em contato com o osso maxila, de forma perpendicular e em sentido ventrodorsal a partir de sua margem alveolar, utilizando como referência o diastema existente entre o terceiro e quarto dentes pré-molares superiores.
Palavras-chave: anestesiologia; morfologia aplicada; odontologia veterinária
Section: Veterinary Medicine
Received
August 22, 2019.
Accepted
January 28, 2020.
Published
March 13, 2020.
www.revistas.ufg.br/vet
visit the website to get the how to cite in the article page.
Introduction
The oral cavity, the associated tissue, and the teeth are structures fundamental for the health of both wild and domestic animals(1). In regard of the region in question, it is known that the sanity of said region is crucial for an efficient processing of food, whilst it is directly related to the maintenance of a good nutritional condition and to increases in the reproductive capacity, in the longevity, and in the quality of life. Still in that context, the presence of oral ailments can affect the animal health in a negative and systemic manner(2), thus causing variable impacts on the wild populations - possible due to these impacts' being limiters of the expectancy of life(3).
In light of the above, the veterinary dentistry poses as one the specialties with the highest potential to significantly contribute for the well-being of animals - especially those which are held in captivity(1). In zoos, as an example, the dental mapping care and treatment have been a highlight over the past decades, on account of their allowing for diagnoses to be performed at an early stage - which can reduce the morbidity and the mortality inherent to those situations(4).
Even in spite of their seeming complex, said clinical procedures are easy to execute in most cases - although they require knowledge of the topographical anatomy and of the numerous therapeutic possibilities which may be applied to the region about to be handled(5). The regional neural block is the most widely used anesthetic technique in animal dental routine(6) and, as such, calls for a constant process of development and fine-tuning for wild patients, as a major part of the techniques resorted to has been devised for domestic specimens. Then, studies which demonstrate methodologies supported by species-specific morphology become a must(7).
Therefore, it has been sought to describe the morphometry of the Hoary Fox's infraorbital foramen with aims at correlating its topography with points of anatomic reference in the skull, thereby providing subsidy for a more effective local anesthetic block in that species.
Materials and methods
Four skulls of adult cadavers of Lycalopex vetulus have been used - two from males and two from females - which belong to the repository for research of the Anatomy Laboratory of the School of Biological Sciences of the Federal University of Goiás, Catalão, Brazil. The preparation of the material was commenced upon the removal of the skin and soft tissue from the region of the head, with the consecutive temporomandibular disarticulation. After that, it has been proceeded to the process of chemical maceration with sodium hydroxide (NaOH, at a concentration of 98% - 99%, Lavitex®, Patos de Minas, Brazil) over 30 minutes for the cleaning of the same, and to a later bleaching by immersion in an aqueous solution of hydrogen peroxide (H2O2, at a concentration of 30% - 36%, Dinâmica®, Indaiatuba, Brazil) with a dilution ration of 1:10, over 30 minutes as well.
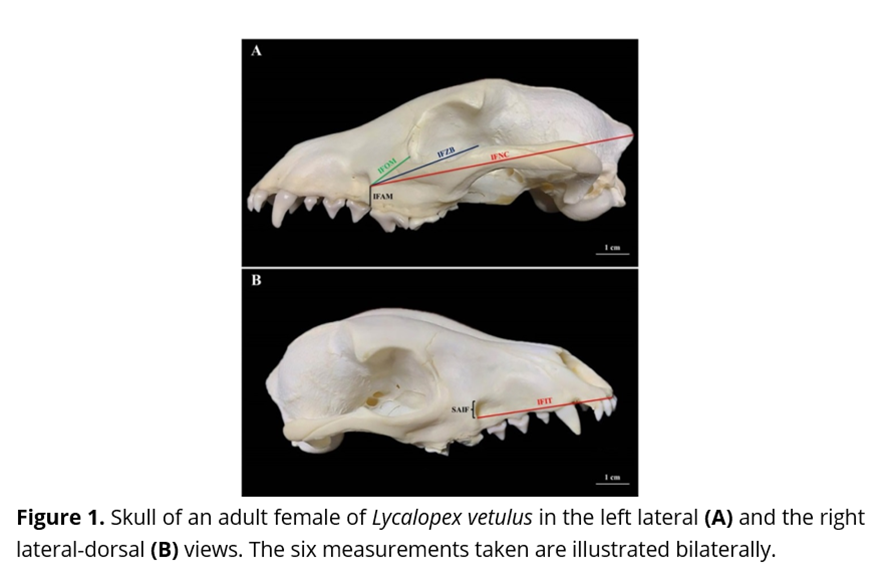
Subsequently, with grounds on the research of Igado(8), Moraes(7) and Maximiano Neto(9), a single examiner - and in duplicate - has carried on with the biometrics proposed on each one of the eight antimeres whilst resorting to a Starrett® digital electronic pachymeter (capacity of 0 – 150 mm, resolution of 0.01 mm, and accuracy of ± 0,02 mm; Itu, Brazil); the same being (Fig. 1): SAIF: length of the sagittal axis of the infraorbital foramen; IFAM: distance between the ventral end of the caudal margin of the infraorbital foramen and the alveolar margin of the maxilla bone in that level; IFOM: distance between the ventral end of the caudal margin of the infraorbital foramen and the orbital margin at the level of the lacrimal foramen; IFZB: distance between the ventral end of the caudal margin of the infraorbital foramen and the dorsal end of the frontal process of the zygomatic bone; IFIT: distance between the ventral end of the caudal margin of the infraorbital foramen and the rostral end of the alveolar margin of the incisive bone in the first upper incisor tooth level; IFNC: distance between the ventral end of the caudal margin of the infraorbital foramen and the caudal end of the nuchal crest at the level of the median sagittal plane.
The numeric data gathered have been subject to a descriptive statistical analysis (arithmetic average, standard deviation, coefficient of variation) and to the student's t test, with a reliability of 95%, by BioEstat® 5.3 software. The anatomic nomenclature used for the description has been in accordance with the International Committee on Veterinary Gross Anatomical Nomenclature(10), and the study has been approved by the Committee of Ethics in the Utilization of Animals of Federal University of Uberlândia (Brazil), protocol n° 81/14.
Results
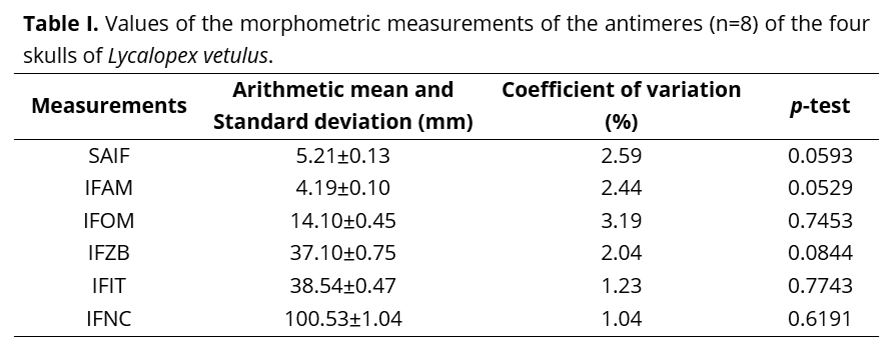
In the totality of the skulls analyzed, the infraorbital foramen was located in the maxilla bone, dorsally-wise from the third upper pre-molar tooth, and, from the ventral end of the caudal margin of the same, it has stood apart from the alveolar margin of that bone (IFAM) by 4.19 ±0.10 mm, in average; from the orbital margin at the level of the lacrimal foramen (IFOM) by 14.10 ±0.45 mm; from the dorsal end of the frontal process of the zygomatic bone (IFZB) by 37.10 ±0.75 mm; from rostral end of the alveolar margin of the first upper incisor tooth (IFIT) by 38.54 ± 0.47 mm; and, from the caudal end of the nuchal crest at the level of the median sagittal plane (IFNC) by 100.53 ±1.04 mm; as it also presented a sagittal axis (SAIF) of 5.21 ±0.13 mm in average.
No statistically significant difference had been observed upon confrontation of the values gathered from the measurements taken from both antimeres, as in demonstrated at Table I.
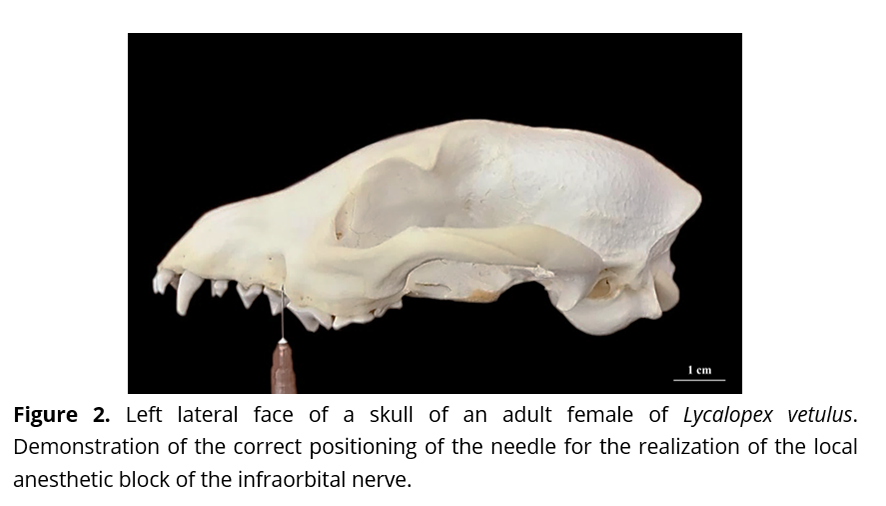
Based on the analyses, it is suggested that the local anesthetic block of the infraorbital nerve can be obtained upon the penetration of the needle by 4.19 mm in contact with the maxilla bone in a perpendicular fashion, and in a ventral-dorsal orientation from the alveolar margin of the same - whilst using, as an anatomic reference, the diastema that exists between the third and the fourth upper pre-molar teeth (Fig. 2).
Discussion
Over the past decades, the management of pain has been an important topic of study - for human and veterinary medicine - and, in that realm, local anesthetic has been resorted to, ever more often, as an adjuvant to the techniques of general anesthesia in dental procedures(11). However, for the administration of the drugs to be more effective - and for the risks of the resulting complications to be mitigated, the anatomic knowledge of the innervation of the oral cavity and of the adjacent structures is of crucial importance(12).
Responsible for the innervation of the teeth(13-16) and the upper lips, the nasal mucosa, the skin of the external nose(14-17) and the local vibrissae(15), the infraorbital nerve represents the final ramification of the maxillary nerve, just as it penetrates the infraorbital canal through the maxillary foramen - still in the interior of the pterygopalatine fossa - and it ends by superficially branching out in the lateral region of the face, as it emerges from the infraorbital foramen(13-16).
In the Hoary Fox, as has been observed by Dyce et al.(18) in domestic animals, by Getty(15), Lantz(17), Lopes and Gioso(11), König and Liebich(16) and Evans and de Lahunta(14) in domestic dogs, and by Maximiano Neto(9) in 36.36% of the specimens of Crab-eating Fox (Cerdocyon thous), the infraorbital foramen was located in the maxilla bone, dorsally-wise from the third upper pre-molar tooth. Yet, in the local dogs of Nigeria, Igado(8) points out the, in spite of that osseous incident's having been positioned in the aforementioned anatomic region, its ventral limit would incline caudally-wise as far as the space that exists between the third and the fourth upper pre-molar teeth - whilst, according to Moraes(7), in the Maned Wolf (Chrysocyon brachyurus), that would be essentially dorsal to those very same two teeth, just as reported by Maximiano Neto(9) in 63.64% of the specimens of Crab-eating Fox.
In a quite ample fashion, this foramen is easily palpable in domestic animals(18) and may admittedly be used as a point of anatomic reference for the local anesthesia of the infraorbital nerve(16), responsible for desensitizing the pulp of the incisors teeth, the fangs, and the first and the second upper pre-molar teeth, the bone and the adjacent soft tissue, the lower eyelid, the upper lip, and the lateral region of the external nose, ipsilaterally. Deeper infiltrations - or with additional dosages - can reach as far as the fourth upper pre-molar tooth(12).
For realization in domestic dogs, the positioning of the needle is recommended in a caudal orientation as from the alveolar mucosa, next to the foramen, until nearly the ventral limit of its aperture(12, 19), or by projecting it as far as the interior of the infraorbital canal - for the purposes of attaining anesthesia in more caudal regions, though not beyond 1 cm(17). In the Maned Wolf, it has been suggested for the a introduction to be conducted through the oral vestibule, in juxtaposition to the lateral face of the alveolar margin of the maxilla bone, and in a vertical orientation, for approximately 10 mm between the third and the fourth upper pre-molar teeth(7), whilst lacking, nonetheless, a consideration of the possible advantages eventually derived from the alteration of the technique.
In a similar fashion to this, for the Hoary Fox, the penetration of the needle is indicated to take place for 4.19 mm in contact with the maxilla bone, in a perpendicular manner and in a ventral-dorsal orientation from the alveolar margin of the same, whilst using, as an anatomic reference, the diastema that exists between the third and the fourth upper pre-molar teeth. In that manner - unlike the application proposed for the domestic dogs where the needle is projects in the direction from which the infraorbital nerve emerges, and as also suggested by Magalhães et al.(20) in the process of improving the anesthetic block of the inferior alveolar nerve of the same species, it is believed that a sharp edge alteration is related only to some extreme points of the subject in question, preserving the functionality of the region and avoiding the occurrence of the neural injuries which may result from the technique.
Among the benefits expected from the perfecting of the same, one can highlight the reductions of the local inflammatory tissue reaction, of the quantity of general anesthetics required, of the central sensitization to pain, and of the employment of drugs in the post-surgery phase(6), thereby avoiding the appearance of hematomas, paresthesia, tissue trauma(11), and focal necrosis(21).
Conclusions
In conclusion, for the realization of the local anesthetic block of the infraorbital nerve of the Hoary Fox, it is indicated for the needle to be introduced approximately by 4.19 mm in contact with the maxilla bone - in a perpendicular fashion and in a ventral-dorsal orientation from the alveolar margin of the same, whilst using, as an anatomic reference, the diastema that exists between the third and the fourth upper pre-molar teeth - thus avoiding the occurrence of the neural injuries which may derive from the technique and preserving, all the while, the functionality of a region that is widely known to be important for the maintenance of the animal health.
Acknowledgements
To the National Council for Scientific and Technological Development (CNPq) and Coordination for the Improvement of Higher Education Personal (CAPES), for the financial support to the authors.
References
1. Pachaly JR. Odontoestomatologia. In: Cubas ZS, Silva JCR, Catão-Dias JL. Tratado de animais selvagens. São Paulo: Roca; 2007. p. 1068-1091. Portuguese.
2. Pachaly JR, Gioso MA. The oral cavity. In: Fowler ME, Cubas ZS. Biology, medicine and surgery of South American wild animals. Ames: Iowa State University Press; 2001. p. 457-463. English.
3. Stimmelmayr R, Maier JAK, Persons K, Battig J. Incisor tooth breakage, enamel defects, and periodontitis in a declining Alaskan moose population. Alces. 2006;42:65-74.
4. Glatt SE, Francl KE, Scheels JL. A survey of current dental problems and treatments of zoo animals. International Zoo Yearbook. 2008;42(1):206-213. https://doi.org/10.1111/j.1748-1090.2007.00032.x
5. Moura AG, Bernardino Júnior R, Severino RS, Teixeira CS. Topografia dos forames mentonianos laterais em suínos das linhagens Agroceres e Seghers Genetics do Brasil. Bioscience Journal. 2006;22(2):119-123.
6. Holmstrom SE, Frost-Fitch P, Eisner ER. Veterinary dental techniques for the small animal practitioner. 3rd ed. Philadelphia: Saunders; 2004. 689p. English.
7. Moraes FM. Morfometria dos forames mandibular, mentual e infraorbital de lobo-guará Chrysocyon brachyurus, Illiger, 1815 aplicada a bloqueios anestésicos. Tese (Doutorado em Ciências Veterinárias), Universidade Federal de Uberlândia; 2016. 55f. https://repositorio.ufu.br/handle/123456789/17588
8. Igado OO. Rostrofacial Indices of the Nigerian Local Dog: Implications in Veterinary Oral and Maxillo-facial Anaesthesiology of the Dolichocephalic Canine Breed. International Journal of Morphology. 2014;32(2):738-743. https://doi.org/10.4067/S0717-95022014000200059
9. Maximiano Neto A. Morfometria do crânio de cachorro-do-mato Cerdocyon thous. Tese (Doutorado em Ciências Veterinárias), Universidade Federal de Uberlândia; 2017. 58f. https://repositorio.ufu.br/handle/123456789/20856
10. International Committee on Veterinary Gross Anatomical Nomenclature. Nomina Anatomica Veterinaria. 6th ed. Hannover, Ghent, Columbia, MO, Rio de Janeiro: Editorial Committee; 2017. 178p. English.
11. Lopes FM, Gioso MA. Anestesia local aplicada à odontologia veterinária. Medvep. 2007;5(14):32-39.
12. Beckman B, Legendre L. Regional nerve blocks for oral surgery in companion animals. Compendium on Continuing Education for the Practising Veterinarian –North American Edition. 2002;24(6):439-444.
13. Budras KD, Mccarthy PH, Fricke W, Richter R, Horowitz A, Berg R. Anatomia do cão: texto e atlas. 5th ed. Barueri: Manole; 2012. 219p. Portuguese.
14. Evans HE, de Lahunta A. Guide to the dissecation of the dog. 8th ed. Saint Louis: Elsevier; 2017. 344p. English.
15. Getty R. Anatomia dos Animais Domésticos. 5th ed. Rio de Janeiro: Guanabara Koogan; 1986. 2000p. Portuguese.
16. König HE, Liebich HG. Anatomia dos Animais Domésticos: texto e atlas colorido. 6th ed. Porto Alegre: Artmed; 2016. 804p. Portuguese.
17. Lantz GC. Regional anesthesia for dentistry and oral surgery. Journal of Veterinary Dentistry. 2003;20(3):181-186. https://doi.org/10.1177/089875640302000306
18. Dyce KM, Sack WO, Wensing CJG. Textbook of Veterinary Anatomy. 4th ed. Saint Louis: Elsevier; 2010. 834p. English.
19. Love L, Egger C. Local and regional anesthesia techniques, Part 3: Blocking the maxillary and mandibular nerves. Vet Med. 2009;104(6):12-15.
20. Magalhães HIR, Romão FB, de Paula YH, Luz MM, Barcelos JB, Silva Z, Carvalho-Barros RA, Ribeiro LA. Morphometry of the mandibular foramen applied to local anesthesia in hoary fox (Lycalopex vetulus). Pesquisa Veterinária Brasileira. 2019;39(4):278-285. https://doi.org/10.1590/1678-5150-PVB-5749
21. McLure HA, Rubin AP. Review of local anaesthetic agents. Minerva Anestesiologica. 2005;71(3):59-74.