DOI: 10.1590/1089-6891v20e-52588
ZOOTECNIA
USE OF NEEM (Azadirachta indica) AS A SUBSTITUTE FOR ANTIMICROBIAL DRUGS IN BROILER CHICKENS’ FEED
UTILIZAÇÃO DE NEEM (Azadirachta indica) EM SUBSTITUIÇÃO AO ANTIMICROBIANO EM RAÇÕES DE FRANGOS DE CORTE
Patricia da Silva Assunção¹ ORCID – http://orcid.org/0000-0001-6506-6615
Heloisa Helena de Carvalho Mello¹ ORCID – http://orcid.org/0000-0002-0312-7424
Alessandra Gimenez Mascarenhas¹ ORCID – http://orcid.org/0000-0001-8333-0723
Maria Auxiliadora Andrade¹ ORCID – http://orcid.org/0000-0002-8373-1671
Karla Andrade Teixeira¹ ORCID – http://orcid.org/0000-0002-1316-9564
Helder Freitas de Oliveira1* ORCID – http://orcid.org/0000-0003-4109-1087
Deborah Pereira Carvalho¹ ORCID – http://orcid.org/0000-0002-7954-235X
¹Universidade Federal de Goiás, Goiânia, GO, Brasil.
*Autor para correspondência - helder@zootecnista.com.br
Abstract
In the present work, we carried out an experiment aiming at evaluating the use of Neem as an antimicrobial substance in the feeds of broiler chickens. We used 240 one-day-old Cobb broiler chicks distributed in an entirely casual design, with 4 treatments, 6 repetitions and 10 birds per experimental unit, during a 21-day experimental period. Treatments consisted of a basal diet with no antimicrobial inclusion, a diet with 0.05% of tylosin, and a diet with 0.05% and 0.1% of Neem. Parameters evaluated were zootechnical performance, feed digestibility, intestinal count of Salmonella sp. and Escherichia coli, and the development of lymphoid organs. Data on the performance and metabolization of nutrients were subjected to an ANOVA and averages were compared with a post-hoc Tukey test considering α=0.05. Data on E. coli counts were analyzed with a Kruskal-Wallis test. Neem did not influence performance variables. The metabolization coefficient of dry matter and feed ethereal extract was better with tylosin (P<0.05). No growth of Salmonella was observed during the experiment. The use of Neem did not reduce E. coli population and had no influence on lymphoid organs’ weight. Therefore, Neem had no effect on the performance, digestibility and intestinal microbiota of birds up to 21 days old.
Keywords: additive, phytogenic additives, bird nutrition, intestinal health
Resumo
Realizou-se um experimento objetivando avaliar a utilização de Neem como antimicrobiano em rações de frangos de corte. Foram utilizados 240 pintos de corte, Cobb, com 1 dia de idade, distribuídos em delineamento inteiramente casualizado, 4 tratamentos, 6 repetições, 10 aves por unidade experimental, o período experimental foi de 21 dias. Os tratamentos consistiram: dieta basal sem inclusão de antimicrobiano; dieta com 0,05% de tilosina; dieta com 0,05% e 0,1% de Neem. Avaliou-se: o desempenho zootécnico, a digestibilidade da ração, contagem intestinal de Salmonella sp. e Escherichia coli, e o desenvolvimento dos órgãos linfoides. Dados de desempenho e metabolização dos nutrientes da ração foram submetidos à ANOVA, médias comparadas pelo teste Tukey, adotou-se α=0,05. Dados de contagem de E. coli foram analisados pelo teste de Kruskal-Wallis. O Neem não influenciou as variáveis de desempenho. O coeficiente de metabolizabilidade da matéria seca e extrato etéreo da ração foi melhor com utilização de tilosina (P<0,05). Não se observou crescimento de Salmonella durante o período experimental. A utilização de Neem não reduziu a população de E. coli, e não influenciou no peso dos órgãos linfoides. A utilização do Neem não afetou o desempenho, digestibilidade e microbiota intestinal das aves até 21 dias.
Palavras-chave: aditivo, aditivos fitogênicos, nutrição de aves, saúde intestinal
Recebido em: 20 de abril de 2018.
Aceito em: 27 de março de 2019.
Introduction
Brazilian broiler production has been one of the most developing farming activities in the last decades, in such a way that strategies for a more efficient production are increasingly being sought. In this context, diet stands out, since it represents the greatest portion of production expenses, and better formulations that provide greater economic profits while maintaining a good zootechnical performance for the birds are necessary.
In order to reach high levels of production and pathogen control for broiler chickens, one of the alternatives is to use feed additives capable of promoting growth. However, some of these products are suffering several restrictions for possessing similar molecules to the ones found in human antibiotics. In this context, several studies have been conducted with natural products with antimicrobial activity to substitute the antibiotics used in these birds’ feed.
Neem (Azadirachta indica A. Juss) is a versatile medicinal plant, as it shows a wide spectrum of biological activity. The chemical composition of its active principals has had considerable progress regarding biological activity and medical applications, thus being considered a valuable source of natural products for the development of medicines, industrial products, and in the integrated management of agricultural plagues(1). In regards to isolated active compounds, salanine, azadirachtin, meliantrol, azadirone, gedunin and nimbolin stand out. Among these, azadirachtin is considered one of the strongest compounds(2). Therefore, Neem has been used in animal feed as a growth promoter due to its distinguished antimicrobial capacity as well as its anticoccidian effect in birds(3). Supporting this, Tipo et al.(4) state that the treatment using Neem as an anticoccidian in broiler chickens was superior to sodium salinomycin.
The objective of the present work was to evaluate the use of Neem (Azadirachta indica) as an antimicrobial in broiler chicken’s feed, aiming at improving intestinal health, nutrient metabolization, and zootechnical performance.
Materials and methods
The experiment was carried out in the experimental aviary at the Universidade Federal de Goiás between May 26 and June 16, 2015. All procedures performed in this study were approved by the Ethics Committee on Animal Use of the Universidade Federal de Goiás (CEUA/UFG), under the protocol 070/14.
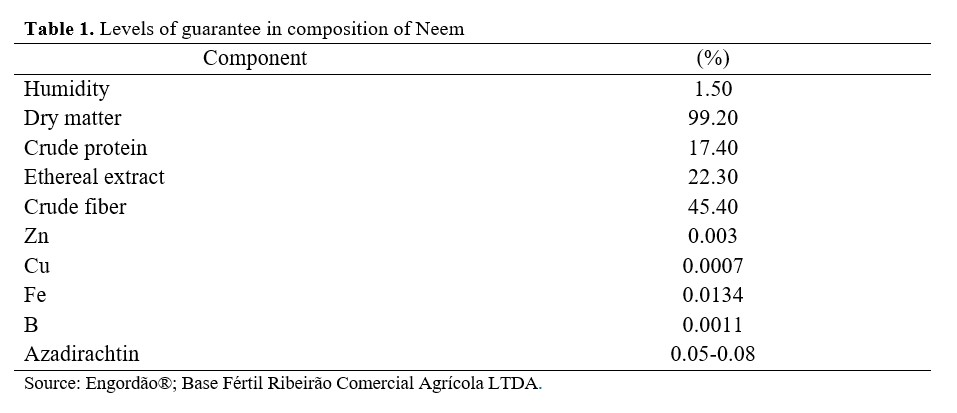
We used 240 one-day-old male broiler chicks from the Cobb lineage. Individuals were distributed in an entirely casual experimental design with four treatments, six repetitions and ten birds per experimental unit. Treatments consisted of four diets based on corn and soybean bran divided in a diet with no antimicrobials, one with 0.05% tylosin, one with 0.05% Neem, and one with 0.1% Neem. We used a commercial tylosin from Elanco® at a concentration of 25% per dose, as recommended by the manufacturer. The Neem used was obtained from the seed residue from the Neem oil extraction and was composed by the crushed, grinded, and dehydrated seeds residue supplied by the producing company (product composition is shown in Table 1)
.
Experimental feeds were formulated to be isonutritive and based on corn and soybean bran to attend the specific nutritional requirements for each phase of poultry raising, according to the recommendations of Rostagno et al.(5). Birds received the experimental diets during a 21-day period divided into two phases: pre-initial and initial (Tables 2 and 3).
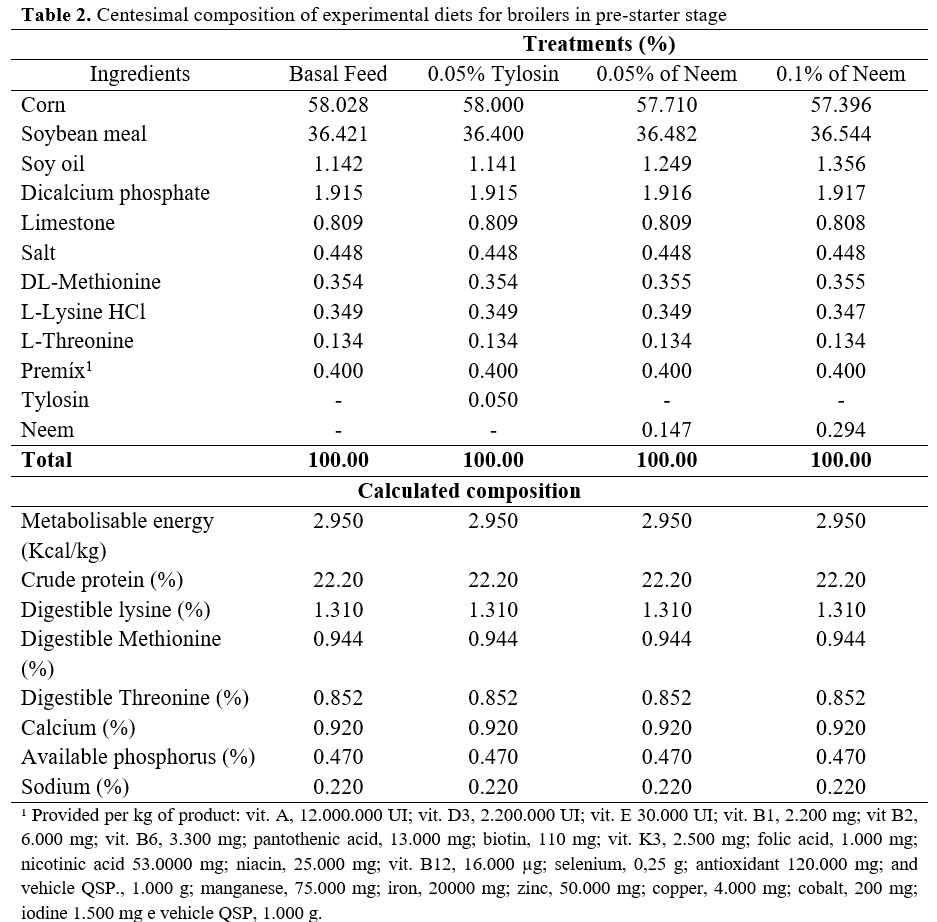
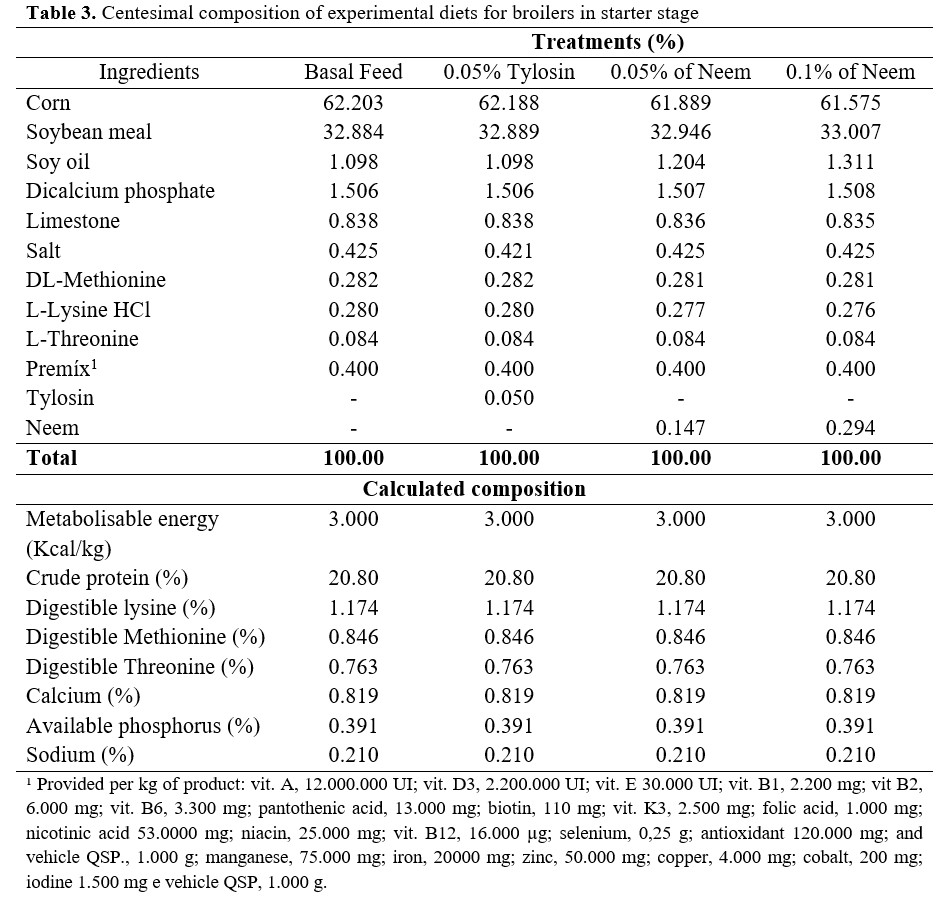
All birds were kept in cages made of galvanized wire with dimensions of 0.5m x 0.4m x 0.4m. Each cage was equipped with a trough water fountain and a feeder in the front, and a tray underneath it to collect excretes.
Birds also received feed and water at will during the entire experimental period, in which the feeders were filled twice a day to avoid wastes. Water was changed and fountains cleaned daily. Temperatures were constantly monitored by thermometers and maximum and minimum temperatures were measured once a day. The environment was controlled with curtains upon necessity.
Bird performance analyses were carried out at the 7 and 21-day-old marks. We evaluated feed consumption (g/bird), weight gain (g/bird), feed conversion, and viability.
At 21 days of age, one bird from each experimental unit was randomly chosen and sacrificed by cervical dislocation after a 4-hour fast. Lymphoid organs (spleen, thymus, and bursa) were collected by using a scalpel and tweezers, and individually weighted in a precision scale to determine immunological activity.
To determine the metabolization coefficient of the feed’s nutrients, a method of total excrete sampling was carried out in the period between 18 to 21 days of age, corresponding to four sampling days. In the experimental period, feed consumption, total excretes produced, and bird weight were measured. Two daily excrete collections (at 8AM and 4PM) were performed, with samples being stored in identified plastic bags and frozen until the end of the collection period.
Samples were than thawed, weighted, homogenized, and subsamples of 400g of excretes were retrieved for laboratory analysis. These were pre-dried in a ventilated stove at 55ºC and subsequently the contents of the dry matter (DM), nitrogen (N), ether extract (EE), and mineral matter (MM) were analyzed following the methods described by Silva and Queiroz(6). In addition, the nutrient metabolization coefficient was calculated by the equations proposed by Sakomura and Rostagno(7).
Cloaca swabs were performed in five birds for each repetition at the 14 and 21-day-old marks. Swabs were put in test tubes with 10 mL of sterile peptone water at 1%, constituting 10-0. Afterwards, 1 mL of peptone water with the sample was transferred, for a 9 mL sterile peptoned saline solution at 0.85%. Serial decimal dilutions were performed until 10-9.
A plating exam was carried out in triplicate with 0.1 mL of different solutions in plates containing bright green agar (BG) and MacConkey agar, which were incubated at 37ºC for 24 hours. After this period, plates were read to observe any growth of colonies suggestive of Salmonella and E. coli in their respective media. If no growth suggestive of Salmonella was found in the BG, 1 mL of the sample in peptone water was transferred to the selenite cystine broth and incubated at 37ºC for 24 hours. Colony-forming units suggestive of E. coli were replicated, three to five, to test tubes with triple sugar-iron (TSI), which were incubated at 37ºC for 24 hours.
The TSI agar that presented characteristics and reactions compatible with E. coli were subjected to the biochemical tests of indol production, urea, H2S production, urease, methyl red and malonate and glucose use in all colony-forming units in the dilutions until 10-9, and were then registered and log-transformed.
Performance and feed nutrient metabolization data were subjected to an analysis of variance (ANOVA) and averages were compared with the Tukey test at a significance level of 5%. E. coli count data were analyzed with the Kruskal-Wallis test at 5%. All statistical analysis were performed in the R software.
Results and discussion
Bird performance was not influenced by the inclusion of neem in the feed (Table 4). These results differ from the ones obtained by Girotto and Santos(8) when evaluating different inclusions of Neem pie in the feed of broiler chickens from 1 to 42 days in the pre-initial and initial phases. The authors observed better results in feed consumption and weight gain in birds that received higher levels (3 to 4%) of Neem pie in the diet.
Similarly, Wankar et al.(9), using Neem leaf powder as a feed supplement for broiler chickens, verified that supplementing 1 to 3 g/kg of product in the feed caused a significant increase in weight gain and a reduction in feed conversion in comparison to birds that did not receive Neem leaf supplementation in the diet.
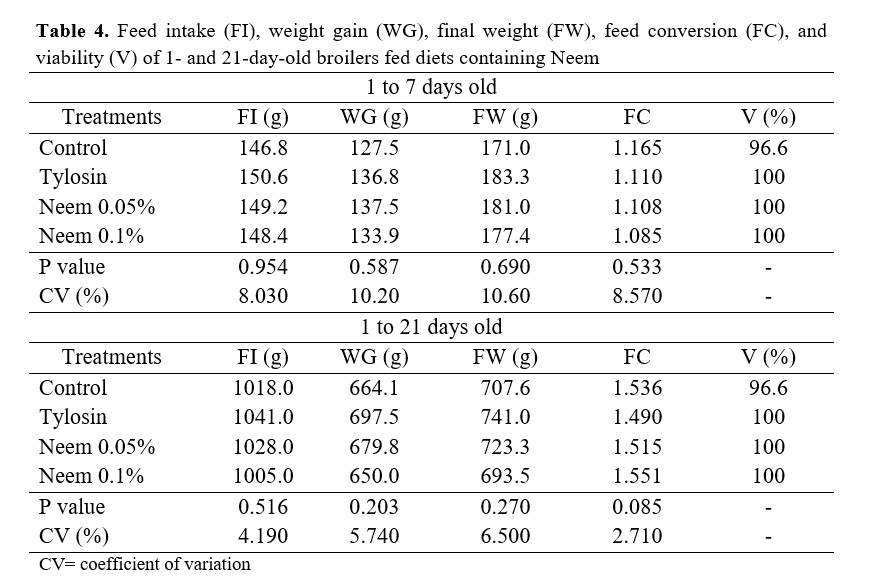
Even though Neem is described in the literature as being used as a growth-promoting antibiotic(10,11,12,13), no alteration in the characteristics evaluated was observed. This might have occurred because sanitary conditions did not result in a significant sanitary challenge, thus no effective action was required by the treatments applied to the birds.
The coefficient of dry matter metabolization and ethereal extract was better with the use of tylosin (Table 5). The coefficient of brute protein metabolization did not significantly differ with the inclusion of Neem in the feed. In regards to the coefficient of mineral matter metabolization, both tylosin and 0.05% Neem in the feed yielded similar results.
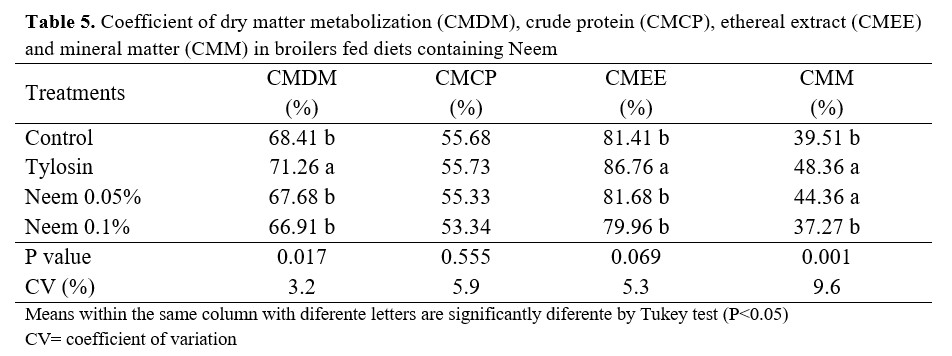
The little diversity in intestinal microbiota in newborn birds, besides being considered as a limiting factor for digestion, also enables intestinal colonization by enteric pathogens. This process’ negative effect has been partly overcome with the use of growth promoters(14). In this way, the results obtained in this research might be related to the action of growth promoters that modify the intestinal microbiota, promoting a stronger equilibrium in the microbe populations, which in turn improves digestion and nutrient absorption.
However, even though the tylosin treatment was superior, the neem inclusion did not affect nutrient digestibility in the feed, since diets with neem did not differ from the ones without antimicrobial substances.
Average values of colony-forming unit (CFU) counts of E. coli expressed in log are shown in Table 6. No Salmonella growth was observed during the experimental period in the media used.
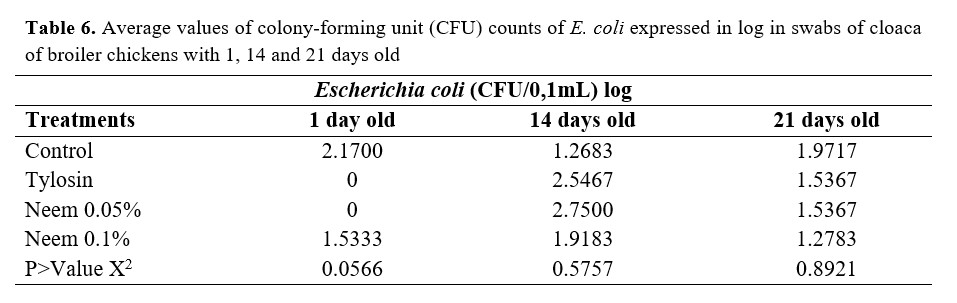
No statistical differences were observed in the CFU counts for E. coli, which suggests that no antimicrobial activity of the Neem pie occurred in any of the days evaluated, since no bacteria reduction existed in the treatments in which the product was included. Similarly, no excessive bacterial increase was observed. These results may indicate that the experimental conditions did not allow us to observe the effects of Neem use, since the antibiotic treatment was compared to the others, including those without Neem.
These results can be supported by Sarker et al.(12), who used Neem leaf as a growth promoter in broiler chickens with colibacillosis and did not observe any antimicrobial activity against E. coli. In addition, there is also the fact that E. coli is a naturally occurring microorganism in healthy birds’ microbiota. Therefore, the results presented show only an equilibrium between the treatments due to the little infestation in the birds, since no sanitary challenge occurred.
Although no antimicrobial activity of the Neem pie was observed in the present study, Odoh and Bratte(15) observed a reduction of enterobacteria in laying hens feces while evaluating the inclusion of several levels of neem dry leaves in these chickens’ feed. They also observed that an inclusion of 10% of these leaves in the feed can be used as an antimicrobial substance and a natural growth-promoting agent in the diets. These results corroborate previous studies that also demonstrated the efficacy of neem leaf extracts against E. coli and S. faecalis(16).
Neem’s antimicrobial activity might be related to the presence of triterpenoids, phenolic compounds, carotenoids, steroids, flavonoids, and azidarachtin(17). However, these compounds are probably present in low quantities in neem pie, which might explain the lack of efficiency of the product as an antimicrobial.
The results of relative spleen, bursa, and thymus weight at the 21-day-old mark are presented in table 7. There was no significant difference between the tested treatments.
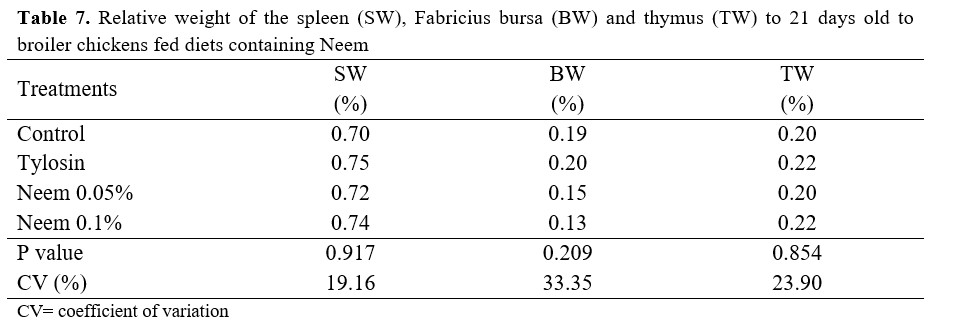
With the results obtained, we may argue that the addition of Neem had no influence on the weight of the lymphoid organs and no interference on the cellular immunity of broiler chickens. This result may be related to the fact that the birds were not severely challenged, thus there was no need for a greater activity of the immune system to contain any kind of infection, since bacterial counts were relatively low.
Conclusion
Neem use did not affect performance, digestibility, and the intestinal microbiota of birds up to 21 days old.
References
1. Hashmat I, Hussain AH, Ahmed A. Neem (Azadirachta indica A. Juss) - A Nature’s Drugstore: An overview. International Research Journal of Biological Sciences. 2012;1(6):76-79. http://www.isca.in/IJBS/Archive/v1/i6/14.ISCA-IRJBS-2012-150.pdf
2. Soglia MC, Osório ACB, Santos Neto C, Fancelli M, Macêdo EF. Nascimento, AS. Usos e aplicações do NIM (Azadirachta indica). EMBRAPA. 2006.
3. Tipu MA, Akhtar MS, Anjum MI, Raja ML. New dimension of medicinal plants as animal feed. Pakistan Veterinary Journal. 2006:26(3):144-148. http://www.pvj.com.pk/pdf-files/26_3/page%20144-148.pdf
4. Tipu MA, Pasha TN, Ali Z. Comparative efficacy of salinomycin sodium and neem fruit (Azadirachta indica) as feed additive anticoccidials in broilers. International Journal of Poultry Science. 2002:1(4):91-93. http://dx.doi.org/10.3923/ijps.2002.91.93
5. Rostagno HS, Albino LFT, Donzele JL, Gomes PC, Oliveira RF, Lopes DC, Ferreira AS, Barreto SLT, Euclides RF. Tabelas brasileiras para aves e suínos: composição de alimentos e exigências nutricionais. Viçosa, Minas Gerais, Editora da Universidade Federal de Viçosa (UFV), 2011;252p.
6. Silva, DJ., Queiroz, AC. Análises de alimentos (métodos químicos e biológicos). 3 ed. Viçosa, Minas Gerais, Editora da Universidade Federal de Viçosa (UFV), 2009;235p.
7. Sakomura, NK., Rostagno, HS. Métodos de pesquisa em nutrição de monogástricos. Jaboticabal, São Paulo, FUNEP, 2007;262p.
8. Girotto VD, Santos GB. Desempenho de frangos de corte de 1 a 42 dias submetidos a diferentes níveis de inclusão da torta de neem (Azadirachta indica) na ração. RETEC. 2012;5(2):67-84. https://www.fatecourinhos.edu.br/retec/index.php/retec/article/view/172
9. Wankar AK, Shirbhate RN, Bahiram KB, Dhenge SA, Jasutkar RA. Effect of Neem (Azadirachta indica) leaf powder supplementation on growth in broilers. Veterinary World. 2009;2(10):396-397. http://www.veterinaryworld.org/Vol.2/October/Effect%20of%20Neem%20(Azadirachta%20Indica)%20leaf%20powder.pdf
10. Durrani FR, Sultan A, Akhtar S, Jan M, Chand N, Durrani Z. Immunomodulatory and growth promoting effects of neem leaves infusion in broiler chicks. Sarhad Journal of Agriculture. 2008;24(4):655-659. https://pdfs.semanticscholar.org/a971/33d25078b150a1571e85c87b526f0a21ba61.pdf
11. Landy N, Ghalamkari G, Toghyan IM. Performance, carcass characteristics, and immunity in broiler chickens fed dietary neem (Azadirachta indica) as alternative for an antibiotic growth promoter. Livestock Science. 2011;142(1):305-309. https://doi.org/10.1016/j.livsci.2011.08.017
12. Sarker SK, Mostofa M, Akter F, Rahman MM, Sultana MR. Effects of aqueous extract of Neem (Azadirachta indica) leaves as growth promoter and anti-colibacillosis in broilers. Bangladesh Journal of Animal Science. 2014;43(2):138-141. http://dx.doi.org/10.3329/bjas.v43i2.20715
13. Mahejabin N, Mostofa M, Akter F, Das S, Alam M. Effects of Neem, turmeric and papaya leaf extract mixture on growth performance of broilers. International Journal of Natural and Social Sciences. 2015;2(2):17-21. http://ijnss.org/wp-content/uploads/2015/02/IJNSS-V2I2-03-pp-17-21.pdf
14. Lorençon L, Nunes RV, Pozza PC, Pozza MSS, Appelt MD, Silva WTM. Utilização de promotores de crescimento para frangos de corte em rações fareladas e peletizadas. Acta Scientiarum. Animal Sciences. 2007:29(2):151-158. http://dx.doi.org/10.4025/actascianimsci.v29i2.219
15. Odoh LI, Bratte L. Effects of varying levels of Neem (Azadirachta indica) leaf meal in layer diets on the haematological and serological indices, and faecal bacterial counts of layers. Journal of Natural Sciences Research. 2015;5(4):37-44. http://www.iiste.org/Journals/index.php/JNSR/article/view/20168/20572
16. Koona S, Budida S. Antibacterial potential of the extracts of the leaves of Azadirachta indica Linn. Notulae Scientia Biologicae. 2011;3(1):65–69. http://dx.doi.org/10.15835/nsb315470
17. Almas K. The antimicrobial effects of extracts Azadirachta indica (Neem) and Salvadora persica (Arak) chewing sticks. Indian Journal of Dental Research. 1999;10(1):23-26. https://www.researchgate.net/publication/12454190_Antimicrobial_effects_of_extracts_of_Azadirachta_indica_Neem_and_Salvadora_persica_Arak_chewing_sticks