DOI:
10.1590/1809-6891v20e-51615
MEDICINA VETERINÁRIA
EFFECT OF BACTERIAL AGENTS OF PORCINE RESPIRATORY DISEASE
COMPLEX ON PRODUCTIVE INDICES AND SLAUGHTER WEIGHT
IMPACTO DE AGENTES BACTERIANOS DO COMPLEXO DE DOENÇAS RESPIRATÓRIAS DE
SUÍNOS(CDRS) NOS ÍNDICES ZOOTÉCNICOS E NO PESO AO ABATE EM SUÍNOS EM
FASE DE TERMINAÇÃO
Talita Brombilla1 ORCID http://orcid.org/0000-0001-6659-7338
Renato Akio Ogata1 ORCID http://orcid.org/0000-0003-1394-0555
Alessandra Figueiredo de Castro Nassar1 ORCID http://orcid.org/0000-0002-9176-0974
Maristela Vasconcellos Cardoso1 ORCID http://orcid.org/0000-0002-9147-170X
Vera Letticie de Azevedo Ruiz2 ORCID http://orcid.org/0000-0002-6983-8923
Claudia Del Fava1* ORCID http://orcid.org/0000-0003-2967-0203
1Centro
de
Pesquisa de Sanidade Animal - Instituto Biológico, São
Paulo,
SP, Brazil
2Faculdade
de Zootecnia e Engenharia de Alimentos - FZEA/USP - Pirassununga, SP,
Brazil
*Corresponding author: delfava@biologico.sp.gov.br
Abstract
Porcine respiratory disease complex comprises the
interaction of two or more infectious agents. The major bacterial
agents involved were investigated in 115 finishing pigs at a farm in
São Paulo State, Brazil: Actinobacillus
pleuropneumoniae (serology, bacterial culture, and multiplex
PCR), Mycoplasma hyopneumoniae
(Mhyo) (nested PCR), Pasteurella
multocida (multiplex PCR), Haemophilus
parasuis (PCR multiplex), and Streptococcus
sp. (bacterial culture). Macroscopic and microscopic lung
lesions were evaluated, and zootechnical indices were recorded. Mhyo
occurred in 113 animals (98.3%), seventeen of which were co-infected
with Streptococcus sp. The
finding of emphysematous lung was associated with significantly lower
final and carcass weight at slaughter. Although vaccinated against
Mhyo with an inactivated immunogen, almost 100% of the animals were
infected. Mhyo infection with and without Streptococcus
sp. co-infection was related to lung lesions of varying degrees and
lower slaughter and carcass weight.
Keywords: differential diagnosis,
Mycoplasma hyopneumoniae,
pleuropneumonia, Streptococcus
sp., swine
Resumo
O
Complexo de Doenças Respiratórias em suínos (CDRS) compreende a
interação de dois ou mais agentes infecciosos, manejo e ambiente. Os
principais agentes bacterianos causadores de CDRS foram investigados em
115 suínos em fase de terminação em uma granja no Estado de São Paulo,
Brasil: Actinobacillus
pleuropneumoniae (sorologia por ELISA, cultivo bacteriano e PCR
multiplex), Mycoplasma
hyopneumoniae (Mhyo) (nested PCR),
Pasteurella multocida (PCR multiplex),
Haemophilus parasuis (PCR multiplex), e Streptococcus
sp (cultivo bacteriano). Foram avaliadas lesões pulmonares
macroscópicas e microscópicas, e impacto nos índices zootécnicos e no
aproveitamento de carcaça. Houve positividade ao Mhyo em 113 animais
(98,26%), destes houve infecção associada ao Streptococcus
sp ao Mhyo em 14,78% (17) animais. O pulmão enfisematoso
diminuiu significativamente o peso no final da terminação e o
peso da carcaça. Apesar de vacinados com imunógeno inativado contra
Mhyo, quase 100% dos animais estavam infectados e as lesões mais
observadas foram broncopneumonia purulenta e pleurite. A infecção de
Mhyo associado ou não ao Streptococcus
sp causou lesões pulmonares em diferentes graus, menor peso ao abate e
da carcaça.
Palavras-chave:
suínos,
pleuropneumonia, diagnóstico diferencial, Streptococcus
sp, Mycoplasma hyopneumoniae.
Received on: February 21st, 2018.
Accepted on: May, 5th, 2019.
Introduction
Respiratory diseases are a critical issue in intensive pig rearing(1). Confined populations are subjected to an environment that may be stressful and reduce immunity, contributing to the occurrence of respiratory disease(2). Pneumonias result in low zootechnical indices, high pharmaceutical costs, and carcass condemnations at the slaughterhouse, where, in Brazil, approximately 50% of animals present some type of lung injury, with these lesions accounting for 50% of all carcass condemnations(3). The more intensive the management system, the higher the costs of production(4).
The etiology of respiratory problems in pigs is complex, usually involving interaction of two or more infectious agents(5), bacterial and/or viral, in addition to factors such as handling and environment, affecting pigs in the growth stages and their value at slaughter(6, 7).
Porcine respiratory disease complex (PRDC) describes a syndrome that encompasses several bacterial agents, including Mycoplasma hyopneumoniae (Mhyo), the agent of enzootic pneumonia in swine; Pasteurella multocida, responsible for pleurites and which, together with Bordetella bronchiseptica, causes atrophic rhinitis; Actinobacillus pleuropneumoniae, causing swine pleuropneumonia; Haemophilus parasuis, the agent of Glasser's disease; and Streptococcus suis, causing pneumonia and pleuritis(7).
Porcine respiratory disease complex is responsible worldwide for economic losses related to mortality and condemnation of slaughtered carcasses(1). Because condemnation has significant impact on profits, research with respect to differential diagnoses of lung lesions could facilitate correlation of lesions with the causal agent to aid in development of disease preventive treatments and cures(1, 8).
In view of the economic losses that respiratory disease represents to pork production, it is essential that the bacterial agents triggering this process be identified. The goal of this work was to determine if bacterial agents of PRDC are related to zootechnical indices of pigs at slaughter.
Materials and methods
This work was approved by the Ethical Committee on Animal Experimentation of the Biological Institute (CETEA-IB) and registered under protocol 130/13 in compliance with the ethical principles in animal experimentation, adopted by the Brazilian Society of Science in Laboratory Animals/Brazilian College of Animal Experimentation (SBCAL/COBEA).
A pork production unit with a full-cycle breeding system was selected from São Paulo State. The breeding was carried out extensively in the gestation phase, semi-intensively in the maternity phase, and was intensive in the other phases.
The animals were crosses of Landrace and Large White breeds and identified by Australian marking and weighed at birth. A balanced feed formulated with Nucleo and Premix (Agrochemical) was provided. Facilities were cleaned and disinfected daily. The piglets were vaccinated with chemically inactivated Mycoplasma hyopneumoniae bacterin (Respisure® One) and/or Circumvent® PCV M (inactivated Mycoplasma hyopneumoniae + porcine circovirus ORF2) and against pasteurellosis, paratyphoid, erysipelas, atrophic rhinitis, leptospirosis, and colibacillosis (Suivem®).
Data of birth and finished weight were collected at the farm, while warm cleaned carcasses were weighed at the slaughterhouse.
At slaughter, samples of whole blood, lung fragments, and mediastinal lymph nodes were collected from 115 pigs. Fragments of lung and mediastinal lymph nodes with macroscopic lesions were divided, with a portion kept refrigerated for bacterial isolation and another fixed in 10% buffered formalin for histology.
For the sero-epidemiological investigation of A. pleuropneumoniae, the ELISA test for antibody detection (IDEXX APP-ApxIV Ab Test, IDEXX Laboratories Inc, USA) was used.
The histological procedures consisted of dehydration,
diaphanization, paraffin embedding, microtomy, and hematoxylin and eosin
staining(9).
Bacteria isolated from lung and lymph nodes samples were suspended in sterile 0.85% saline solution, and 1 mL of the suspension was added to 3 mL brain heart infusion broth for enrichment. Ten μL of the enriched suspension was seeded in 5% sheep blood agar and incubated for 48 hours at 37°C. In samples demonstrating bacterial growth, morphological characteristics of colonies including size, shape, color, and presence and type of hemolysis were recorded. Colonies were Gram stained and examined microscopically for morphology and Gram staining characteristics(10). Bacterial species were identified by specific biochemical tests(10, 11). Identification of Streptococcus was to genus.
Multiplex PCR was used to detect A. pleuropneumoniae, P. multocida, and H. parasuis, and nested PCR for Mhyo. The DNA extraction was performed on lung and lymph node samples using a commercial kit (Quick-gDNA™ MiniPrep, Uncapped Columns, Zymo Research, USA).
For the confirmation of molecular analyses, DNA extraction was conducted by PCR for the β-actin gene(12). This protocol uses swine-specific primers (actin F-TGAGACCTTCAACACGCC/actin R-ATCTGCTGGAAGGTGGAC), with a size of 745 base pairs (bp). For the PCR reaction, 2.5 μL of extracted DNA was added to 22.5 μL PCR Mix containing 1.25 U Taq DNA polymerase, 200 μM of each deoxynucleotide, 1X PCR Buffer (20 mM Tris-HCl, pH 8.4, 50 mM KCl), 0.75 mM MgCl2, and 10 pmol of each primer. The amplification process was carried out in a thermocycler under the following conditions: 95°C for 5 minutes; 39 cycles of 95°C for 30 seconds, 56°C for 45 seconds, and 72°C for 45 seconds; and a final extension of 72ºC for 5 minutes.
In the multiplex PCR, specific primers used for each agent were A. pleuropneumoniae AP-IVF: ATA CGG TTA ATG GCG GTA ATG G/AP-IVR, ACC TGA GTG CTC ACC AAC G (13); P. multocida KMT1 T7: ATC CGC TAT TTA CCC, AGT GG/KMT1 SP6: GCT GTA AAC GAA CTC GCC AC (14); and H. parasuis HPS-F: GTG ATG AGG AAG GGT GGT, GT/HPS-R: GGC TTC GTC ACC CTC TGT (15) amplifying a segment of 346, 460, and 821 bp, respectively. These primers were used in multiplex PCR as in Hričínová et al.(16).
As a positive control of A. pleuropneumoniae, standard samples of serotypes I, III, and lyophilized Va were used (Empresa Irfa Química e Biotecnologia Industrial Ltda)(17). For positive control of H. parasuis a sample from the Department of Veterinary Preventive Medicine and Animal Health, FMVZ USP, São Paulo, SP, Brazil was used, and P. multocida strain INCQS 00096 (ATCC 6530) was obtained from the Reference Microorganism Collection in Sanitary Surveillance, CRMVS, FIOCRUZ-INCQS, Rio de Janeiro, RJ. The negative sample was ultra-pure water. Samples were amplified using 10 μL extracted DNA plus 40 μL of the reagent mixture containing 1.25 U Taq DNA polymerase, 1X PCR Buffer (10 mM Tris-HCl, pH 8.0, 50 mM KCl), 200 μM of each deoxynucleotide, 2 mM MgCl2, and 30 pmol of each primer. The amplification process was performed in a thermocycler under the following conditions: denaturing at 95°C for 5 minutes followed by 29 cycles of 94°C for 30 seconds, 58°C for 30 seconds, and 72°C for 45 seconds; followed by final extension at 72°C for 7 minutes.
The nested PCR for Mhyo used external primers A-F: GAGCCTTCAAGCTTCACCAAGA/B-R: GTGTTAGTGACTTTTGCCACC (18) and internal C-F: ACTAGATAGGAAATGCTCTAGT/D-R: GTGGACTACCAGGGTATCT(19) with fragments of 649 bp and 352 bp, respectively. For the PCR, the reagent solution of the mix consisted of 1.0 U Taq DNA polymerase; 10 pmol of each primer (Mhyo A-F and Mhyo B-R); 200 μM of each dNTP; 0.75 mM MgCl2; 1X PCR Buffer (20 mM Tris-HCl pH 8.4, 50 mM KCl), and 5 μL extracted DNA. Mhyo DNA (Department of Preventive Veterinary Medicine and Animal Health, FMVZ USP, São Paulo, SP, Brazil) was positive control, and the negative control was ultra-pure water. Conditions were 95°C for 3 minutes followed by 35 cycles of 95°C for 1 minute, 64°C for 1 minute, 72°C for 90 seconds, and a final extension of 72°C for 5 minutes. In the nested PCR, the reagent solution was identical to PCR, replacing only the primers (Mhyo C-F and Mhyo D-R) and reducing the hybridization temperature from 64°C to 60°C.
For biomolecular reactions, the amplified products were examined under ultraviolet light (UV) transillumination after GelRed™ stained 1% agarose gel electrophoresis. The amplified fragments were compared to the molecular size standard (100 bp, DNA Ladder, Invitrogen) arranged in the gel with the analyzed samples. The gel image under UV light was recorded in PhotoDocumentor coupled to a computer.
The analysis of variance (ANOVA) considered mixed infection of Streptococcus sp. and Mhyo and Mhyo alone. We evaluated the zootechnical variables birth weight (PN), weight gain to slaughter (GMD), final weight (PF), and carcass weight (PCAR) relative to sex, presence/absence of disease, age in days, and macro- and micro-scopic lesions. Data were analyzed in a completely randomized design. The mixed model was used and the random effects of batch and residue were analyzed with the MIXED procedure in SAS v. 9.3(20). When significant, the means of zootechnical variables were compared using Fisher’s least significant difference test (DIFF option of the LSMEANS command). In all analyses, the significance was declared at p≤0.05.
Results and discussion
ELISA serology is simple and highly sensitive(21). None of the pigs showed A. pleuropneumoniae antibodies. Some animals may present negative serology and carry A. pleuropneumoniae in the nasal cavities and tonsils without showing clinical signs, evidence that the agent can colonize the upper respiratory tract without inducing seroconversion(22, 23). For this reason, bacteriological analyses were conducted, also resulting in no PCR isolation or detection of A. pleuropneumoniae.
Multiplex PCR was negative for A. pleuropneumoniae, P. multocida, and H. parasuis(15), and no animals presented macroscopic lung lesions characteristic of these diseases. This may have been due to the sanitation practices of the farm.
Bacterial genera were isolated from lung and lymph node samples (Table 1), including the only genus with significance to respiratory diseases in swine, Streptococcus, with 17 of the 115 (14.8%) animals positive. Diagnosis by bacteriological culture is a challenge due to the extensive use of antibiotics to control disease in pigs and may be a major reason for lack of isolation of bacterial strains involved in respiratory problems. High antibiotic use may increase the occurrence of bacterial resistance to antimicrobials.
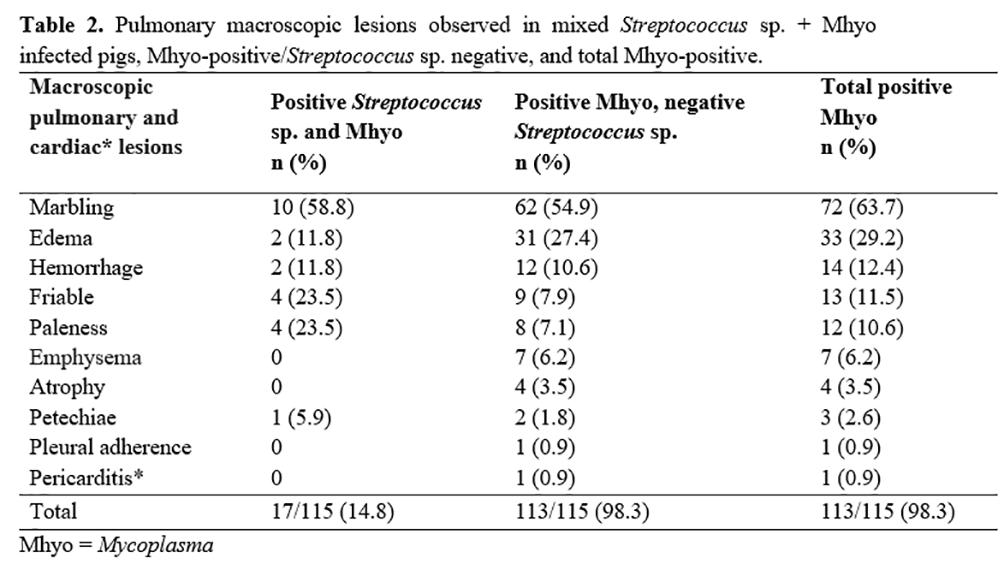
Table 2 summarizes macroscopic lesions in lung of the 17 pigs positive for both Streptococcus sp. and Mhyo pathogens.
Macroscopic lesions lung and heart of the 113 Mhyo-positive animals were consistent with those previously reported associated with Mhyo(2, 8, 24) (Table 2).
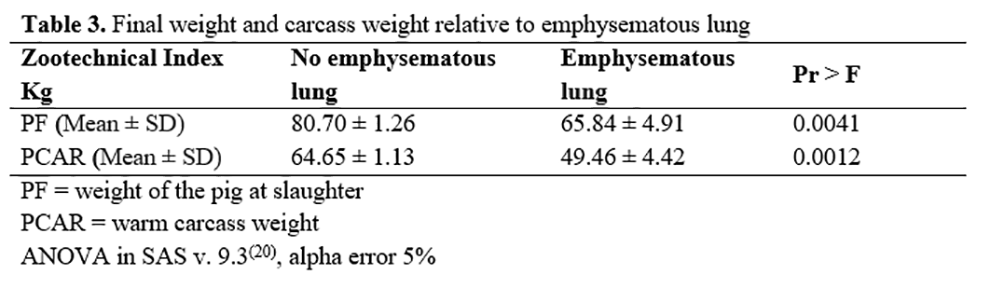
Mean PF (65.84 ± 4.91kg) (p=0.0041) and PCAR (49.46 ± 4.42kg) (p=0.0012) was significantly lower in the animals that presented pulmonary emphysema compared to animals without macroscopic lesions in lungs (PF 80.70 kg and PCAR 64.65 kg) (Table 3). Other macroscopic lesions showed no correlation with performance.
Adequate respiratory capacity is essential for the growth and fattening of pigs(25), with respiratory disorders being one of the most important problems in modern pork production due to the economic loss caused by mortality and condemnation of carcasses(1).
The main microscopic lesions in the 17 pigs with mixed Streptococcus sp. x Mhyo infection are presented in Table 4.
In the Mhyo-positive animals, the predominant microscopic lesions were consistent with Mhyo as described by other authors(8, 24) (Table 4).
Macroscopic and microscopic examination demonstrated Mhyo association with pulmonary lesions with and without co-infection of Streptococcus sp. Mhyo was identified by nested PCR in 98.26% of the pigs with mild lung lesions and those with moderate to severe lung lesions and pericarditis. Since the pigs were vaccinated with the inactivated Mhyo pathogen as piglets, the presence of Mhyo DNA may indicate sub-clinical infection (colonization) in cases of minimal lung lesions, with the agent present in insufficient quantities to cause disease(26). This result demonstrated the presence of active infection on the farm, since many pigs vaccinated for Mhyo presented compatible lesions, including one animal showing adherence of the pleura and pericardium in the costal gradil, serious chronic lesions.
Mhyo causes enzootic pneumonia of swine, a catarrhal bronchopneumonia considered one of the major infectious diseases in pigs worldwide and causing significant economic losses(29). Mhyo control consists of biosecurity measures and vaccination. Vaccination is the primary control measure recommended for enzootic pneumonia, but immunization may fail due to differences between the vaccine and the field strains. The commercial vaccines available in Brazil consist of inactivated whole Mhyo cells of a strain isolated in England, and it is questionable whether this strain has similar characteristics to those circulating in Brazilian swine herds and in other parts of the world(30,31). These vaccines have been shown effective in reducing clinical signs but confer only partial protection against the development of lesions and transmission of the agent. They do not prevent colonization of Mhyo in the respiratory tract, as observed in the present study, and the vaccine efficacy is in question(27). The infection pressure may reach the point at which the vaccine does not generate sufficient immunity to neutralize the infection. A study in Minas Gerais, Brazil demonstrated the genetic diversity of Mhyo in field samples from pig lungs(28). The samples were categorized into 30 genetic groups showing broad distribution in the studied regions. Histology identified lesions suggestive of enzootic pneumonia in swine. The results indicate that several variants of Mhyo circulate in swine herds, suggesting its broad genetic diversity even within a specific region. The authors concluded that it is necessary to investigate protective antigens and strategies in the elaboration of vaccine prototypes against Mhyo infection.
Molecular techniques have demonstrated high genetic diversity(30-36), proteomic variation(37), and differences in virulence of Mhyo strains(38).
Weight gain at slaughter, PF, and PCAR were significantly higher (p=≤0.05) in the pigs with mixed Streptococcus sp. and Mhyo (PCAR) infection compared to Mhyo only, while there was no association with PN (Table 5). The mean PN of the animals with mixed infection was 1.91 kg and of animals positive only for Mhyo was 1.75 kg. The low number of pigs without Mhyo infection in the studied facility did not allow analysis of variance to compare the productive indices of a negative group with the animals infected by one or both agents.
Embrapa(39) established the zootechnical indices of mean weight of piglet at birth 1.4 kg, ideal >1.5 kg; mean weight of pigs at slaughter (133 days), 78 kg, ideal >83 kg; at 140 days >85 kg, ideal >90 kg; at 147 days 92 kg, ideal >97 kg; at 154 days 98 kg, ideal >103 kg.
Both the Mhyo- and Mhyo + Streptococcus sp.-positive
animals presented PF (mean 137 days) consistent with Embrapa’s criteria(39).
The studied farm did not operate as an intensive system of commercial
production on an industrial scale. Embrapa(39) did not
discuss pathogens causing PRDC in the farms evaluated.
We were able to verify that the vaccine against swine mycoplasmosis was not effective in the prevention of Mhyo infection or of chronic lesions. The development of effective immunogens and diagnostic tests using strains circulating in Brazil is essential.
The prevalence and economic impact of respiratory disease in pigs was evaluated in 62 farms in the states of Rio Grande do Sul, Santa Catarina, and Paraná(25) with examination of 3,788 pigs for the frequency and extent of pneumonia, and 3837 heads for frequency and severity of destruction of the nasal turbinates. Pneumonia was diagnosed in 2079 (54.9%) animals, and atrophic rhinitis was recorded in 1894 (49.4%), with a mild degree of injury in 42.6% of cases of pneumonia and 32.4% in atrophic rhinitis. Although most of the pigs did not show obvious clinical signs, there was a reduction in average daily weight gain of 6% with atrophic rhinitis and of 3 to 8% with pneumonia, demonstrating, as in our study, the impact of respiratory diseases on productivity.
In a study of clinical cases of respiratory disease in finished pigs(40), animals with respiratory clinical signs were necropsied for macroscopic evaluation and for histological and microbiological analysis. Bacterial isolation was performed for bacteria Mycoplasma hyorhinis and immunohistochemistry for Influenza A, swine circovirus type 2, and Mycoplasma hyopneumoniae. Suppurative bronchopneumonia and pleuritis were the main respiratory lesions found, and Mycoplasma hyopneumoniae and Pasteurella multocida type A were the most prevalent pathogens. Pasteurella multocida type A was associated with a greater extent of lung lesions. In 58% of samples, more than one infectious agent was identified, showing the importance of multiple agents in the endemism of respiratory disease in pigs.
Multiple pathogens increase the challenge of controlling infections, and it is essential to identify agents and jointly evaluate pathology, etiology, and clinical status of finished pigs. The quantification of production losses is necessary to support the development of prophylactic measures in the farms(40, 41).
Conclusion
The presence of Mhyo with or without Streptococcus sp. was sufficient to cause macroscopic and microscopic lesions and lead to productive losses related to slaughter weight and carcass weight.
Acknowledgements
Thanks for Talita Brombilla's Masters funding by the Coordination of Higher Education Personnel (code 001) and by the Foundation for Research Support of São Paulo State (2013/07964-5). To Mr Lindolfo Rocha, from ABASE Comércio e Representações Ltda., Jaguariúna, SP, Brazil, for provision of the ELISA App Iddexx kit. To Profa Dr Andrea Mike Moreno of the Department of Veterinary Preventive and Animal Health of FMVZ USP, São Paulo, SP, Brazil, for providing reference strains of Haemophilus parasuis and Mycoplasma hyopneumoniae. To Irfa Química e Biotecnologia Industrial Ltda, for standard samples of A. pleuropneumoniae serotypes I, III and Va. Thanks to Dr Joslayne Noely Gonçalves Cirillo, of the Centro Avançado de Pesquisa Tecnológica do Agronegócio de Bovinos de Corte – Instituto de Zootecnia - Sertãozinho, SP, Brazil, for statistical analyses.
References
1.
Paladino ES, Gabardo MP, Lunardi PN, Morés N, Guedes RMC. Anatomopathological pneumonic aspects associated with
highly pathogenic Pasteurella multocida in finishing pigs. Pesquisa
Veterinária Brasileira. 2017;37(10):1091-1100. Portughese.
2. Alberton GC, Mores MAZ. Interpretação de lesões no abate como
ferramenta de diagnóstico das doenças respiratórias dos suínos. Acta
Scientiae Veterinariae. 2008;36(1):95-99. Portughese.
3. Krabbe EL, Santos Filho JI, Miele M, Martins FM. Embrapa Suínos e
Aves. Tópicos atuais na produção de suínos e aves: Cadeias produtivas de
suínos e aves. Pelotas: Instituto Federal Sul-rio-grandense; 2013. p. 9.
Portughese.
4. Christensen G, Sorensen V, Mousing J. Diseases of the respiratory
system. In: Straw BE, D’Allaire S, Mengeling WI et al., ed. Diseases of
swine. 8th ed. Ames: Iowa State University Press; 1999. p. 913-940.
5. Sorensen V, Jorsal SE, Mousing J. Diseases of the
respiratory system. In: Zimmerman JJ, D'Allaire S, Taylor DJ. (ed).
Diseases of swine. 9th ed. Oxford: Blackwell Publishing, 2006.
p.149-177.
6. Thacker EL. Immunology of the porcine respiratory disease complex.
Veterinary Clinics of North America-Food Animal Practice.
2001;17(5):551-565.
7. Hansen MS, Pors SE, Jensen HE, Bille-Hansen V, Bisgaard M, Flachs EM,
Nielsen OL. An investigation of the Pathology and Pathogens Associated
with Porcine Respiratory Disease Complex in Denmark. Journal of Clinical
Pathology. 2010;143(2-3):120-131.
8. Mores MAZ, Donin DG, Cestari FK, Alberton GC. Achados
patológicos e bacteriológicos em lesões pulmonares responsáveis por
condenações de carcaças de suínos. Archives of Veterinary Science. 2016;21(4):92-100.
Portughese.
9. Prophet EB, Mills B, Arrington J B, Sobin LH (Orgs.) Métodos
Histotécnicos. Washington: Registro de Patologia de los Estados Unidos
de América y Instituto de Patología de las Fuerzas Armadas de los
Estados Unidos de América, 1995. 280p. Spanish.
10. Quinn PJ, Markey BK, Carter ME, Donnelly WJ, Leonard FC. Microbiologia
Veterinária e Doenças Infecciosas. 1.ed. Porto Alegre: Artemed Editora
SA, 2005. Portughese.
11. Koneman EW, William MJ, Schreckenberger PC, Winn WC, Allen SD, Woods
GL Diagnóstico Microbiológico: texto e atlas colorido. 6. ed. Rio de
Janeiro: Guanabara Koogan, 2008.1565p. Portughese.
12. Hui RKH, Zeng F, Chan CMN, Yuen KY, Peiris JSM, Leung FCC. Reverse Transcriptase PCR Diagnostic Assay for the
Coronavirus Associated with Severe Acute Respiratory Syndrome. Journal
of Clinical Microbiology. 2004;42(5):p.1994-1999.
13. Xiao GS, Cao SJ, Duan LL, Wen XT, Ma XP, Chen HM. Identification and
detection of Actinobacillus pleuropneumoniae in infected and
subclinically infected pigs by multiplex PCR based on the genes ApxIVA
and OmIA. Agricultural Sciences in China. 2006;5(2):146-154.
14. Townsend KM, Frost AJ, Lee CHW, Papadimitriou JM, Dawkins HJ.
Development of PCR assays for species- and type- specific identification
of Pasteurella multocida isolates. Journal of Clinical Microbiology.
1998;36(4):1096-1100.
15. Oliveira S, Galina L, Pijoan C. Development of a PCR test to
diagnose Haemophilus parasuis infections. Journal of Veterinary
Diagnostic Investigation. 2001;13(6):495-501.
16. Hričínová M, Holoda E, Mudroňová D, Ondrašovičová S. Multiplex PCR
Assay for Detection of Actinobacillus pleuropneumoniae, Pasteurella
multocida and Haemophilus parasuis in lungs of pigs from a
slaughterhouse. Folia
Microbiologica. 2010;55(6):635-640.
17. Ferraz MICP, Ferreira DR, Goes AC, Gregori F, Miyashiro S, Ruiz VLA.
Detecção direta de Actinobacillus pleuropneumoniae em órgãos de suídeos
do Estado de São Paulo pela técnica de reação em cadeia pela polimerase
(Nested-PCR). Arquivos do Instituto Biológico. 2010; 77(1):143-148.
Portughese.
18. Mattsson JG, Bergstrom K, Wallgreen P, Johansson KE. Detection of
Mycoplasma hyopneumoniae in nose swabs from pigs by in vitro
amplification of the 16S rRNA gene. Journal of Clinical Microbiology.
1995;33(4):893-897.
19. Casalmiglia M, Pijoan C, Bosch GJ. Profiling Mycoplasma
hyopneumoniae in farms using serology and a nested PCR technique. Swine
Health and Production. 1999;7(6):263-268.
20. SAS Institute Inc. SAS® 9.3 Statements: Reference. Cary, NC: SAS
Institute Inc., 2011.
21. Gottschalk M, De Lasalle F, Radacovici S, Dubreuil JD. Evaluation of
long-chain lipopolysaccharides (LPS-CL) of Actinobacillus
pleuropneumoniae serotype 5 for the serodiagnosis of swine
pleuropneumonia. Veterinary Microbiology. 1994;38:315-327.
22. Sidibé M, Messier S, Lariviere S, Gottschalk M, Mittal KR. Detection
of Actinobacillus pleuropneumoniae in the upper respiratory tract as a
complement to serological tests. Canadian Journal of Veterinary
Research. 1993;57(3):204-208.
23. Chiers K, Donné E, Van Overbeke I, Ducatelle R, Haesebrouck F.
Actinobacillus pleuropneumoniae infections in closed swine herds:
infection patterns and serological profiles. Veterinary
Microbiology.
2002;85(4):343-352.
24. Sobestiansky J, Barcellos DESN, Mores N, Oliveira SJ, Carvalho LFOS,
Moreno AM, Roehe PM. Clínica e patologia suína. 2ed., Goiânia, 1999. 464
p. Portughese.
25. Sobestiansky J, Dalla Costa O, Mores N, Bariorini Junior W, Piffer
IA, Guzzo R. Estudos ecopatológicos das doenças respiratórias dos
suínos: prevalência e impacto econômico em sistemas de produção dos
estados de Santa Catarina, Rio Grande do Sul e Paraná. Comunicado
técnico: Embrapa, 2001. 6 p. [acesso 2014 Jan 12]. Disponível em:
https://www.embrapa.br/busca-de-publicacoes/-/publicacao/439780/estudos-ecopatologicos-das-doencas-respiratorias-dos-suinos-prevalencia-e-impacto-economico-em-sistemas-de-producao-dos-estados-de-santa-catarina-rio-grande-do-sul-e-parana.
Portughese.
26. Tamiozzo PJ, Pelliza BR, Carranza AI, Ambrogi A. Monitoramento da
presença de Mycoplasma hyopneumoniae em granjas de suínos durante a
implementação de programas de erradicação. Ciencia Rural. 2011;41(4):699-705. Portughese.
27. Haesebrouck F, Pasmans F, Chiers K, Maes D, Ducatelle, R, Decostere
A. Efficacy of vaccines against bacterial diseases in swine: what can we
expect? Veterinary
Microbiology. 2004 ;100(3-4):255-268.
28. Moreira TS, Marques HZ, Souza LFL, Araújo EM, Assao VS, Santos MR,
Moreira MAS, Silva Júnior A. Perfil Genético de Mycoplasma hyopneumoniae
no Estado de Minas Gerais, Brasil. In: Oliveira LG, Oliveira MEF,
Mechler ML (Ed.). Anais do II Simpósio Internacional de Produção e
Sanidade de Suínos. Jaboticabal: FCAV UNESP, 2017. p.172-175. ISSN
978-85-7805-170-9. ePDF . [acesso Out 2017]. Disponível em:
https://www.simpork.com/trabalhos-cientificos. Portughese.
29. Thacker, E. L.; Minion, F. C. Mycoplasmosis. In: Zimmerman, J.J;
Karriker, L. A.; Ramirez, A. et al. (Eds.) Diseases of swine. 10th ed.
Ames: Iowa State University Press;2012. p.779-797.
30. Vranckx K, Maes D, Calus D, Villarreal I, Pasmans F, Haesebrouck F.
Multiple-locus variable-number tandem-repeat analysis is a suitable tool
for differentiation of Mycoplasma hyopneumoniae strains without
cultivation. Journal of Clinical Microbiology. 2011;49(5):2020-2023.
31. Stakenborg T, Vicca J, Maes D, Peeters J, De Kruif A, Haesebrouck F,
Butaye P. Comparison of molecular techniques for the typing of
Mycoplasma hyopneumoniae isolates. Journal of Microbiological
Methods.2006;66(2):263-275.
32. Dos Santos LF, Sreevatsan S, Torremorell M, Moreira MA, Sibila M,
Pieters M. Genotype distribution of Mycoplasma hyopneumoniae in swine
herds from different geographical regions. Veterinary Microbiology.
2015;175(2-4):374-381.
33. Charlebois A, Marois-Créhan C, Hélie P, Gagnon CA, Gottschalk M,
Archambault M. Genetic diversity of Mycoplasma hyopneumoniae isolates of
abattoir pigs. Veterinary Microbiology. 2014;168(2-4):348-356.
34. Nathues H, Beilage EG, Kreienbrock L, Rosengarten R, Spergser J.
RAPD and VNTR analyses demonstrate genotypic heterogeneity of Mycoplasma
hyopneumoniae isolates from pigs housed in a region with high pig
density. Veterinary Microbiology. 2011;152(3-4):338-345.
35. Strait EL, Madsen ML, Minion FC, Christopher-Hennings J, Dammen M,
Jones KR, Thacker EL. Real-time PCR assays to address genetic diversity
among strains of Mycoplasma hyopneumoniae. Journal of Clinical
Microbiology. 2008;46(8):2491-2498.
36. Dubosson CR, Conzelmann C, Miserez R, Boerlin P, Frey J, Zimmermann
W, Häni H, Kuhnert P. Development of two real-time PCR assays for the
detection of Mycoplasma hyopneumoniae in clinical samples. Veterinary
Microbiology. 2004;102(1-2):55-65.
37. Calus D, Baele M, Meyns T, De Kruif A, Butaye P, Decostere A,
Haesebrouck F, Maes D. Protein variability among Mycoplasma
hyopneumoniae isolates. Veterinary Microbiology. 2007;120(3-4):284-291.
38. Vicca J, Stakenborg T, Maes D, Butaye P, Peeters J, De Kruif A,
Haesebrouck F. Evaluation of virulence of Mycoplasma hyopneumoniae field
isolates. Veterinary
Microbiology. 2003;97(3-4):177-190.
39. EMBRAPA - Empresa Brasileira de Pesquisa Agropecuária. Boas práticas
de produção de suínos. 2006. Disponível em: http://www.cnpsa.embrapa.br.
Acessado em: 22 nov. 2014. Portughese.
40. Morés MAZ, Oliveira Filho JX, Rebelatto R, Klein CS, Barcellos DEN,
Coldebella A, Morés N. Aspectos patológicos e microbiológicos das
doenças respiratórias em suínos de terminação no Brasil. Pesquisa
Veterinária Brasileira. 2015;35(8):725-733.Portughese.
41. Alberton GC, Rocha DL. Como diagnosticar corretamente as doenças
respiratórias dos suínos na recria e na terminação. Acta Scientiae
Veterinariae. 2010;38:29-35. Portughese.