DOI: 10.1590/1089-6891v20e-38059
ZOOTECNIA
EFFECT OF PRE-SLAUGHTER CONFINEMENT STRESS ON PHYSICOCHEMICAL PARAMETERS OF CHICKEN MEAT
EFEITO DO ESTRESSE NO CONFINAMENTO PRÉ-ABATE SOBRE OS PARÂMETROS FÍSICO-QUÍMICOS DA CARNE DE FRANGO
Cesar Lazaro¹* ORCID – http://orcid.org/0000-0003-4856-0034
Carlos Adam Conte-Junior² ORCID –http://orcid.org/0000-0001-6133-5080
Martín Medina-Vara³ ORCID – http://orcid.org/0000-0003-3341-7876
Daniel Mota-Rojas³ ORCID – http://orcid.org/0000-0003-0562-0367
Rosy Cruz-Monterrosa⁴ ORCID – http://orcid.org/0000-0003-2859-013X
Isabel Guerrero-Legarreta⁵ ORCID – http://orcid.org/0000-0003-3113-8814
¹Universidad Nacional Mayor de San Marcos, Lima, Peru.
²Universidade Federal Fluminense, Niterói, RJ, Brazil.
³Universidad Autónoma Metropolitana-Xochimilco, México D.F., Mexico.
⁴Universidad Autónoma Metropolitana-Lerma, Lerma de Villada, E.M., Mexico.
⁵Universidad Autónoma Metropolitana-Iztapalapa, México D.F., México.
*Correspondent author - clazarod@unmsm.edu.pe
Abstract
The effect of pre-slaughter lairage stress on biogenic amines, pH, and color with the CIELab system (where three variables L*, a*, b* are estimated: L* for lightness, a* defines redness, and b* defines yellowness) in chicken breast meat was investigated. Before slaughtering, 25 broilers were selected and divided into five groups according to lairage holding time (30 min or 3 h), day/night schedule (daylight or nighttime) and control (no lairage). After slaughtering, breasts (M. Pectoralis major) were removed, stored for 9 days at 4 °C, and analyzed every 3 days. The results showed a pH decrease during nighttime holding. Also, a three-hour pre-slaughter holding (daytime or night) resulted in high biogenic amine concentration, increase in lightness (L*), and reduction in redness (a*) during storage. Cadaverine concentration showed a rapid increase from day 6 onward. The time and schedule of chicken lairage is a pre-slaughter stress factor that affects meat quality. Based on these observations, it is recommended to slaughter chickens immediately on arrival at the processing plants.
Keywords: biogenic amines, lairage, poultry meat, storage, stress.
Resumo
Foi avaliado o efeito do estresse produzido pelo tempo de espera pré-abate sobre a produção de aminas biogênicas, pH e cor pelo sistema CIELab (onde três variáveis L*, a*, b* foram determinadas: L* indica a luminosidade, a* indica o vermelho e b* determina o amarelo) na carne de peito de frango. Antes do abate 25 frangos de carne foram selecionados e divididos em cinco grupos conforme o tempo de espera pré-abate (30 min ou 3 h), programação dia/noite (diurno ou noturno) e controle (sem tempo de espera). Após abate, os peitos (M. Pectoralis major) foram separados, estocados por 9 dias a 4°C, e analisados cada 3 dias. Os resultados evidenciaram uma redução do pH nas aves que aguardaram o abate na noite. Além disso durante a estocagem incrementou-se os valores de aminas biogênicas e da luminosidade (L*) e houve redução dos teores de vermelhos (a*) no tempo de espera de três horas (diurno ou noturno). As concentrações da cadaverina incrementaram-se a partir do dia 6 de estocagem. O tempo e horário de espera em frangos de carne pode é um fator de estresse pré-abate que afeta a qualidade da carne. Contudo, é recomendável realizar o abate dos frangos imediatamente após de sua chegada ao centro de abate.
Palavras chave: Aminas biogênicas, tempo de espera, carne de frango, estresse
Received on: October, 15th, 2015
Accepted on: March, 29th, 2019
Introduction
Pre-slaughter phase in the production process of the chicken meat industry has potentially important implications for animal welfare. Potential stressors such as handling, noise, vibration, and thermal variations during the transport, feed and water withdrawal, unfamiliar environments, high density in crates, and lairage time could predispose damage and even death, possibly more so when flocks are in poor health(1).
Chickens may be temporarily held in lairage before slaughtering to recover from transport stress. However, an improper holding period can be a major factor in lowering meat quality. Lairage periods longer than 2 or 4 h alter the metabolism, cause hyperglycemia, hypercalcemia, hyperlactatemia, hyperkalemia, hyponatremia, acidosis, severe dehydration, and modifies the physical parameters in chicken meat(2,3). Sometimes, a continuous processing line in slaughterhouses involves a longer lairage time to maintain the animal stock before slaughtering. In this case, methods to ensure animal welfare are essential. In fact, the quality of poultry meat is a result of complex interactions between genotype and environment, mainly in relation to stress severity before slaughtering(4).
Meat quality is frequently evaluated only by microbial indicators or sensory characteristics. However, the use of chemical indicators, such as volatile bases and acids, nucleotide breakdown, and biogenic amines, is a more precise method(5). These compounds are a result of enzymatic decarboxylation of specific free amino acids. Prerequisites for the formation of amines in foods are the availability of free amino acids, the presence of decarboxylase-positive microorganisms, and favorable conditions for microbial growth and decarboxylase activity. Therefore, decarboxylation can be largely prevented by controlling bacterial growth through regulating temperature, oxygen availability, redox potential, and pH(6, 5, 7-9).
Knowing the mechanisms of production and control of biogenic amines is important for assessing public-health hazards. Consuming foods containing high concentrations of biogenic amines can induce adverse reactions such as headaches, nausea, palpitations, and rashes. Likewise, carcinogenic effects of nitrosamines, compounds formed by the interaction of biogenic amines with nitrites, have been reported(10). Therefore, detection of biogenic amines in the food industry is a valuable tool for assessing freshness and quality of a wide variety of protein-containing products. This study evaluated the effect of preslaughter lairage stress on biogenic amine concentration, pH, and color of chicken breast meat.
Material and methods
The experiment received ethical approval (Code number MC-2014/1-43) from the Institutional Committee on the Care and Use of Animals of the Universidad Nacional Autónoma de México in Mexico City. Broilers (Ross) were reared on a commercial farm located in central Mexico (2649 m above sea level), 18 °C mean ambient temperature and 68% relative humidity (RH). During the final rearing period, the birds were fed with 18% crude protein, 3225 kcal/kg gross energy. At the end of the rearing period and before slaughter, a total of 25 commercial female chickens (49 days old and 3 kg live weight on average) were taken and placed randomly in crates. Each crate held 5 birds on 0.55 m² (0.11 m²/bird). Five groups (four treatments and one control) were performed. Treatments consisted of antemortem stress based on two lairage time during day (08:00) or night (20:00) slaughtering process, as follows: 30 min lairage in daylight (D30); 180 min lairage in daylight (D180); 30 min lairage during nighttime (N30); and 180 min lairage during nighttime (N180). Chickens in the control group (C) were slaughtered within 10 min from the catching. The slaughtering process was carried out on the farm in order to avoid the stress of transport. Stunning was carried out by cervical dislocation and killing by neck severing, in accordance with Mexican Regulations for Animal Slaughtering(11). Finally, the carcasses were manually eviscerated, washed, labeled by group, and transported to the laboratory under refrigerating conditions (4 °C) for analysis. All these actions were performed in over 3 separate trial days according the daylight, nighttime, and control groups.
Upon arrival at the laboratory from the farm (within 2 hours after slaughtering), both sides of the breast (M. Pectoralis major) were removed from each carcass (10 breasts per group), cut into sections, and stored at 4 °C for 9 days. Instrumental color, pH, and concentration of biogenic amines (putrescine, cadaverine, and histamine) were analyzed at 0, 3, 6, and 9 days of storage. Meat pH was analyzed by a HI 99163 portable penetration potentiometer (Hanna®) equipped with an FC 232D electrode (Hanna®).
Instrumental color was measured in terms of CIELab, in which L* (lightness), a* (redness), and b* (yellowness) were measured using a ColorFlex™ colorimeter (Hunter-Lab, Reston, VA) with a D65 reference illuminant and a view angle of 10°(12). Measurements were performed on the upper (ventral) side of the Pectoralis major muscle from different locations on the muscle surface.
Biogenic amines were determined by liquid chromatography. In order to obtain linear calibration curves, regression equations, and coefficients of determination (r²), standard solutions (10 g/L) were prepared with 18.24 mg putrescine dihydrochloride (C4H12N2 2HCl), 17.14 mg cadaverine dihydrochloride (C5H14N2 2HCl), and 16.57 mg histamine dihydrochloride (C5H9N3 2HCl) in 1 mL 0.1 N HCl (Sigma-Aldrich, St. Louis, MO) and stored at 4 °C until use. Working solutions (0.2, 0.4, 0.8, 1.6, 3.1, 6.3, 12.5, 25, 50, and 100 mg/L) were obtained by diluting the stock solution.
The concentration of biogenic amines in meat samples was determined according to the method reported by Lázaro et al.(7). In brief, the meat was treated with 5% perchloric acid, using NaOH to neutralize the system and derivatized with benzoyl chloride (40 µL). The reaction was stopped with 5 M NaCl(13). The mixture was extracted with diethyl ether and evaporated to dryness with a nitrogen stream. Finally, the residue was dissolved in 1000 µL acetonitrile:water (50:50) and stored at 4 °C. Identification and quantification were carried out using a Varian HPLC ProStar system, fitted with a ProStar 210 solvent delivery module. Control and data analysis were carried out using the software Galaxy (version 1.9). Chromatographic separations were achieved using a reversed-phase HPLC column C18 (Beckman Ultrasphere ODS, 250 mm x 4.6 mm i.d., 5 µm) and guard column (Waters Symmetry C18, 3.9 x 20 mm). The mobile phase for gradient elution consisted of two solvent systems: acetonitrile (solvent A) and MilliQ water (solvent B). The gradient elution was carried out as follows: 50% solvent A for 12 min; solvent A was increased to 100% over 5 min; and finally returned to 50% over 3 min. Other operation conditions were: 1 mL/min flow rate, 254 nm detection wavelength, 20 µL injection volume, and 25 °C column temperature. The eluent was monitored by a UV-Vis Prostar 325 detector. The final result was a mean of three injections of each sample.
For this trial, a total of 25 chicken were selected and divided into 5 groups. Two sides of the breast (M. Pectoralis major) were removed from each chicken, cut in 6 portions of approximately 125 g (30 breast portions for each group), and stored at 4 °C for 9 days. Sampling was carried out on days 0, 3, 6, and 9 of storage, with 5 samples each (replicates). All analyses were performed in triplicate, except for the color, which had four replications. Data were subjected to analysis of variance (ANOVA) and Tukey's multiple range tests using the SAS program adapted to a personal computer(14). Differences were considered to be significant at P ≤ 0.05. The statistical model included the effect of day/night schedule (daylight or night), holding time (30 or 180 min), and storage time (0, 3, 6, and 9 days). The response variables were: pH, instrumental color (L*, a*, and b*), and concentration of putrescine, cadaverine, and histamine.
Results and discussion
In Table 1, a decrease was observed on day 3 in groups subjected to daytime stress (D30 and D180), with a subsequent significant increase (P<0.001). Nighttime holding (N30 and N180) produced an even steeper decrease, with the lowest pH (5.16) observed for N180.
The rate of the decease of pH and ultimate pH were probably related to stress levels in holding during nighttime(15). In our study, a rapid pH decline was observed for nighttime treatments as a result of bird stress conditions immediately before slaughter. These results were in agreement with Ngoka and Froning(16), who reported a steep pH decline in meat from turkeys that struggled in the shackle line. Warriss et al.(17) evaluated the consequences of vibration during the transport (180 min) and found a significant reduction of pH levels in white and red chicken muscle. On the other hand, Rodrigues et al.(2) determined that different lairage times (2, 4, and 6 hours) in the slaughterhouse did not affect the initial pH of chicken breast meat (P>0.05). Nannan et al.(18) determined that actions like showering and ventilation before slaughtering slowed down postmortem glycolysis and prevented glycogen from breaking rapidly. In the present study, the pH increased in all treatments after 9 days of storage due to protein and peptide depletion and amine production. These results are in agreement with those found by Surmei and Usturoi(19), who reported a pH increase from 5.87 to 6.38 in chicken breast after 10 days of storage.
The L*, a*, and b* values available in Table 1 show that after slaughtering, groups N30 and D180 had the lowest and highest L* values. Otherwise, a longer holding time (180 min) significantly increased L* values throughout storage (P<0.001), whereas L* value had non-significant variations during storage in 30-min holding treatments (P>0.01). These results suggest that L* values increase in chickens submitted to longer holding time. Zhu et al.(20) evaluated and classified chicken meat as PSE-like muscle (L*>53) and normal muscle (L*=48 to 53) to assess meat quality. Our results showed an L* < 46 in all groups (after slaughtering and during storage). However, the results must be interpreted with caution because several factors (colorimeter, feed, sex, age, environmental, stress, etc.) can affect the final values. Contrasting results on the relationship between pre-slaughter stress in poultry and L* values have been reported. Debutet al.(21) found that the pH in the muscle from birds placed in a room at 35 °C for 120 min before slaughtering was reduced, but L* values were not affected. Bianchi et al.(22) showed that longer holding periods (>6 h) produced L* = 52.12 when compared to shorter periods (<6 h), which showed an L* = 52.84.
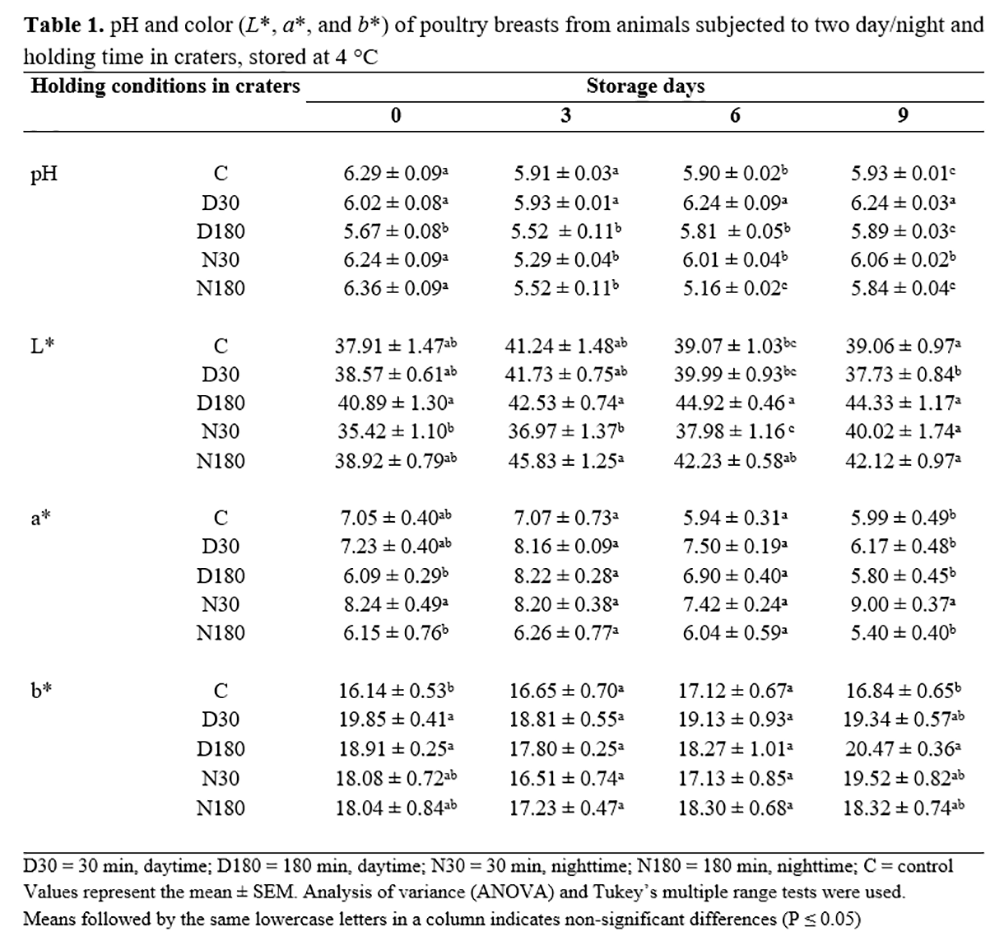
In this study, C, D30, and N30 groups had a higher red component (a*) when compared to D180 and N180 after slaughtering. This result shows the effect of longer holding time (3 h) in reducing a* values. During storage, C, D30, D180, and N180 groups showed a similar trend. Debut et al.(4) concluded that high a* is a result of rapid pH decrease in meat. However, our results do not agree with theirs because groups with high a* values also showed high pH levels. On the other hand, the yellow component (b*) mostly showed non-significant differences among treatments and during storage. Our results were similar to those of Lázaro et al.(23), who found b* values in a range of 10 to 16 for the same period of storage, but higher than those found by Qiao et al.(24), who reported b* values of 6 to 9. Variations in meat color could be related to differences in chicken breeds, diet, housing, management, colorimeter calibration, location of measurement, etc. However, Garcia et al.(25) reported that b* values in chicken meat were not influenced by stress factors before slaughtering.
According to Mota-Rojas et al.(15), color is directly related to the final pH; a paler appearance results from final low pH in meat, whereas darker meat is related to high pH. Nannan et al.(18) indicated that a faster glycolytic rate would lead to a high lactate accumulation in a short time during the postmortem period, and then the pH value decreases rapidly, resulting in lower ultimate pH value, paler color, and poor water-holding capacity in breast meat. The reason to explain these changes was most likely related to muscle protein denaturation.
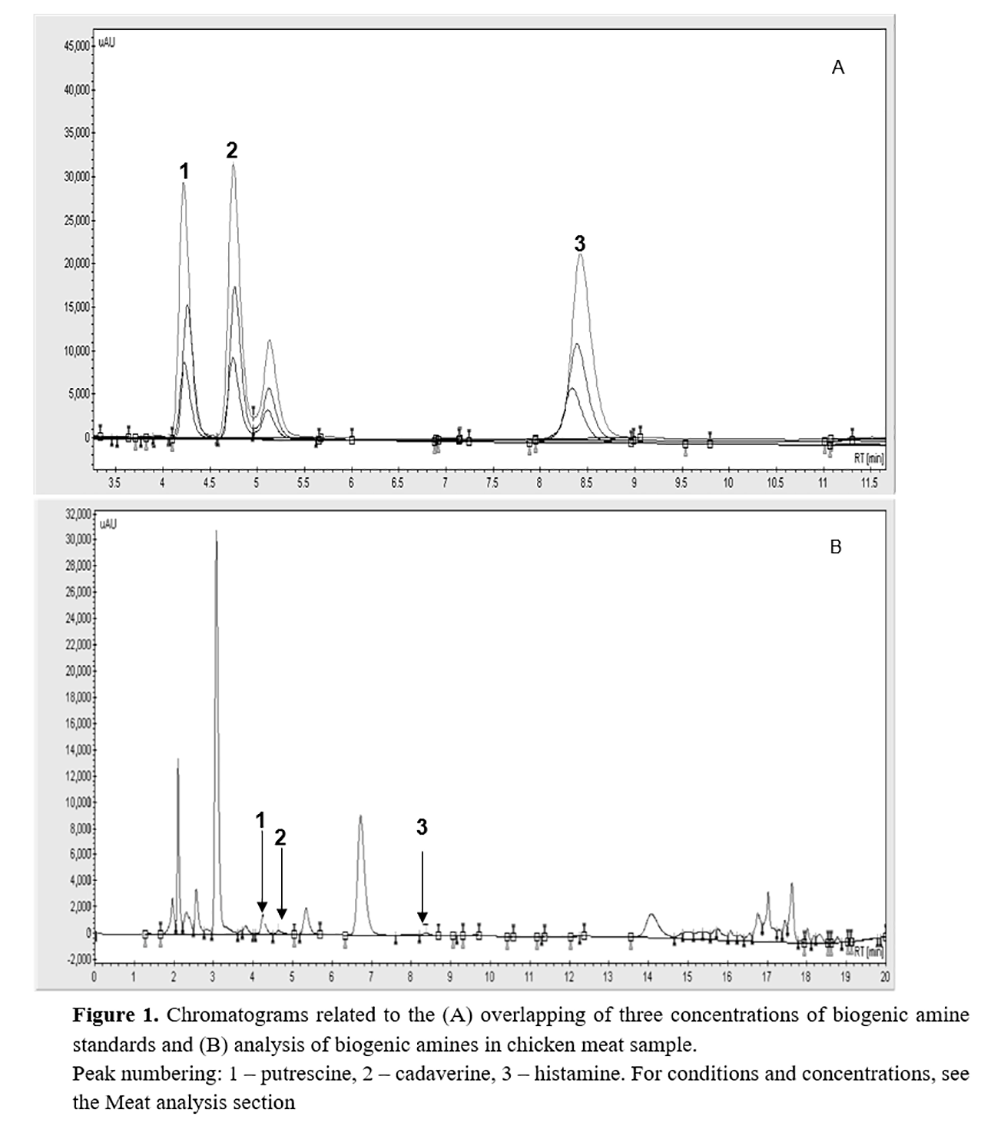
Chromatograms identified biogenic amine (Fig. 1). The absence of minor peaks indicated that these compounds were efficiently separated from other meat components by the extraction method described above. Our results are in agreement with those of Lázaro et al.(7), who found a similar efficiency in peak separation for putrescine and cadaverine when applying the same chromatographic method.
About the concentration of biogenic amines in the meat during storage (Table 2), the results suggest that lairage time affected the cadaverine levels. After slaughtering, meat from birds held for 30 min in lairage (D30 and N30) showed concentrations below 1.0 mg/kg, followed by a slight increase during storage and reaching levels that did not exceed 4 mg/kg. On the other hand, groups held in lairage for 180 min (D180 and N180) began with levels above 1.0 mg/kg, followed by a slight increase to below 2.0 and 5.0 mg/kg, respectively, until day 6, and finally reached levels between 12 and 14 mg/kg on day 9. In contrast, the control group, slaughtered immediately without previous crating, showed a similar trend to D180 and N180 treatments.
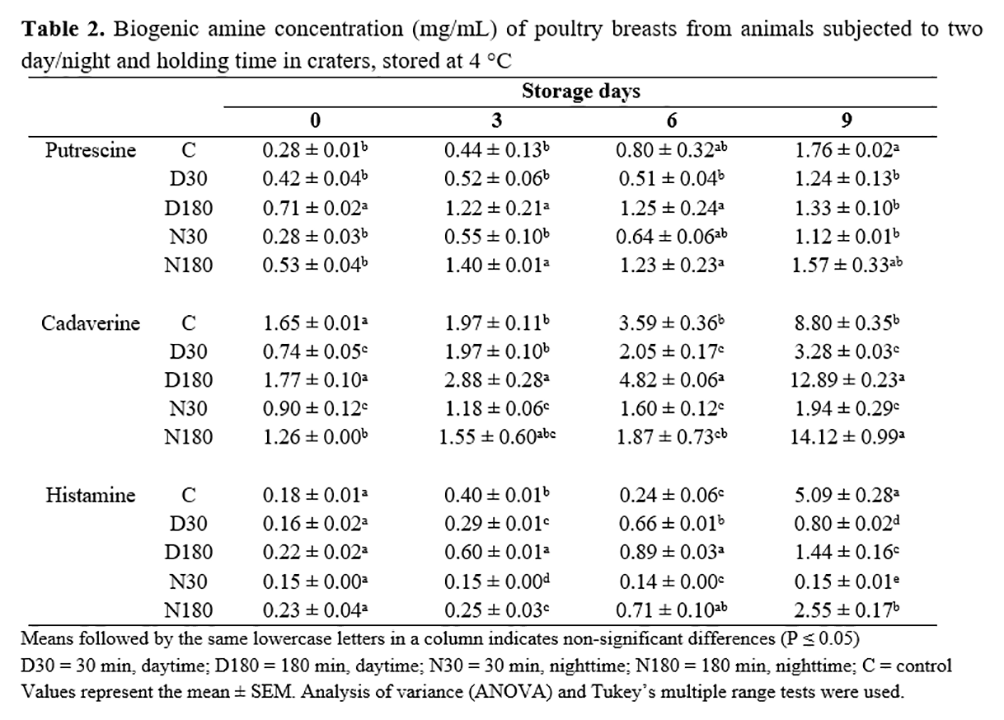
At the beginning of this study, all groups showed putrescine levels below 0.50 mg/kg, with no significant differences (P>0.01), except for D180 (180 min, daytime), which showed the highest level (0.71 mg/kg). A gradual and slight increase was observed from day 3 onward for C, D30, and N30 groups, reaching concentrations in a range of 1.12 to 1.76 mg/kg. Groups D180 and N180 rapidly increased to 1.22 and 1.40 mg/kg, respectively, on day 3, and afterward did not change significantly (P>0.01) during storage.
Except for N30, which had no significant variations during storage (P>0.01), histamine concentrations in all groups gradually increased, more strongly from day 6 onward. At the end of storage, C group showed the highest histamine concentrations (5.09 mg/kg). Treatments D30, D180, and N180 showed histamine levels of 0.80, 1.44, and 2.55 mg/kg, respectively.
Some authors(26, 6, 5, 27) have reported the usefulness of biogenic amines as quality indicators during storage, and there seems to be a close relationship with biochemical and bacteriological parameters. Our results for cadaverine, putrescine, and histamine at 24 h after slaughtering were rather low in comparison to those reported by Sander et al.(28), who found 200 and 500 mg/kg for putrescine and cadaverine, respectively, in chicken carcasses. Kozová et al.(29) reported putrescine levels of around 1.0 mg/kg in chicken breast meat. On the other hand, Rokka et al.(30) found putrescine and cadaverine concentrations below 10 mg/kg during one week of storage at 4 to 8 °C, and Silva and Gloria(31) did not detect putrescine, cadaverine or histamine during the first ten days of storage at 4 °C, but a rapid increase occurred on day 15, reaching values of 20.4, 4.3, and 10.3 mg/kg for each amine.
Environmental stressors such as catching, crating, and transport led to changes in muscle and meat metabolite concentrations(3). Energy exhaustion is responsible for the ultimate properties of meat(32). PSE meat has a lower final pH than normal, promoting protein breakdown (presence of free amino acids) and providing favorable conditions for bacterial growth(33). Lairage time is considered a stress factor that can affect chicken meat quality and accelerate the production of biogenic amines. In this experiment, the longer crating duration (D180 and N180) resulted in increases in putrescine and cadaverine levels during storage, which suggested that these two biogenic amines could be useful indicators for chicken meat freshness.
No definite evidence is available regarding the direct effect of in vivo stressors on biogenic amine production in chicken meat. Özogul and Özogul(34) determined that slaughtering rainbow trout by suffocation in ice slurry (stress method) increased the levels of putrescine and cadaverine compared to slaughtering by percussion (conventional method). On the other hand, Cruz-Monterrosas et al.(35) suggested that bruised areas in meat promote its fast decomposition and bacteria growth. This fact favored an increment of biogenic amine concentrations in beef meat. These results emphasized the importance of implementing best management practices during pre-slaughter operations to reduce a possible risk factor. However, according to Jacobs et al.(1), although lairage time is an important stressor, it is not the unique factor that affects meat quality and even the death in the pre-slaughter phase.
Conclusion
In conclusion, longer holding times resulted in paler and less-yellow meat, with a low pH and high cadaverine concentrations from day 6 onward regardless of the schedule (daylight/nighttime). These results show the importance of reducing bird stress before slaughtering. Based on these observations, it would seem desirable that chickens should be killed immediately on arrival at the processing plants, and the recommended lairage time is no more than one hour.
Acknowledgments
The authors thank the Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (process no. E-26/103.003/2012, FAPERJ, Brazil) and the Conselho Nacional de Desenvolvimento Científico e Tecnológico (processes no. 311361/2013-7, 313917/2013-2 and 401922/2013-8, CNPq, Brazil) for the financial support. We also thank the Ministerio de Educación del Perú, Oficina de Becas y Crédito Educativo (OBEC) and the Agencia Mexicana de Cooperación Internacional para el Desarrollo for the academic collaboration fellowship.
References
1. Jacobs L, Delezie E, Duchateau L, Goethals K, Tuyttens FAM. 2017. Broiler chickens dead on arrival: associated risk factors and welfare indicators. Poultry Science 96:259-65. https://doi.org/10.3382/ps/pew353
2. Rodrigues DR, Café MB, Jardim Filho RM, Oliveira E, Trentin TC, Martins DB et al. Metabolism of broilers subjected to different lairage times at the abattoir and its relationship with broiler meat quality. Arquivo Brasileiro de Medicina Veterinária e Zootecnia. 2017;69:733-41.http://dx.doi.org/10.1590/1678-4162-9268.
3. Roldan-Santiago P, Mota-Rojas D, Guerreo-Legarreta I, Mora-Medina P, Borderas-Tordesillas F, Alarcon-Rojo AD et al. Animal welfare of barrows with different antemortem lairage times without food. Veterinární Medicína. 2013;58(6):305-11. http://vri.cz/docs/vetmed/58-6-305.pdf
4. Debut M, Berri C, Arnould C, Guemené D, Santé-Lhoutellier V, Sellier N et al. Behavioural and physiological responses of three chicken breeds to pre-slaughter shackling and acute heat stress. British Poultry Science. 2005;46(5):527-35. http://dx.doi.org/10.1080/00071660500303032
5. Halász A, Baráth Á, Simon-Sarkadi L, Holzapfel W. Biogenic amines and their production by microorganisms in food. Trends in Food Science & Technology. 1994;5(2):42-9. https://doi.org/10.1016/0924-2244(94)90070-1
6. Lázaro CA, Conte-Júnior CA, Canto ACVCS, Monteiro MLG, Costa-Lima BRC, Mársico ET et al. Biochemical changes in alternative poultry meat during refrigerated storage. Revista Brasileira de Ciência Veterinaria. 2012;19(3):195-200. http://dx.doi.org/10.4322/rbcv.2014.108
7. Lázaro CA, Conte-Júnior CA, Cunha FL, Mársico ET, Mano SB, Franco RM. Validation of an HPLC Methodology for the Identification and Quantification of Biogenic Amines in Chicken Meat. Food Anal Methods. 2013:1-9. http://dx.doi.org/10.1007/s12161-013-9565-0.
8. Fraqueza MJ, Alfaia CM, Barreto AS. Biogenic amine formation in turkey meat under modified atmosphere packaging with extended shelf life: Index of freshness. Poultry Science. 2012;91(6):1465-72. http://dx.doi.org/10.3382/ps.2011-01577.
9. Lázaro CA, Conte-Júnior CA. Chromatographic methods for biogenic amines determination in foods of animal origin. Brazilian Journal of Veterinary Research and Animal Science 2013;50(6):430-46. http://dx.doi.org/10.11606/issn.1678-4456.v50i6p430-446
10. Ladero V, Calles M, Fernández M, Álvarez MA. Toxicological effects of dietary biogenic amines. Current Nutrition & Food Science 2010;6:145-56. http://dx.doi.org/10.2174/157340110791233256
11. Mexico. Norma Oficial Mexicana: Especificaciones técnicas para la producción, cuidado y uso de los animales de laboratorio (NOM-062-ZOO-1999). Diario Oficial de la Federación. 1999 6 de diciembre de 1999. http://www.dof.gob.mx/nota_detalle.php?codigo=762506&fecha=22/08/2001
12. AMSA. Meat color measurement guidelines. Champaign, IL, USA: American Meat Science Association; 2012.
13. Signorini ML, Guerrero-Legarreta I. Producción de aminas biogénicas en carne de bovino conservada con ácido láctico de origen químico y bacteriano. Revista mexicana de ingeniería química. 2009;8(1):41-9. http://www.scielo.org.mx/scielo.php?script=sci_arttext&pid=S1665-27382009000100004&lng=es&tlng=es.
14. SAS. Statistical Analysis Systems User's Guide. Statistics Version 9.1. . Cary, NC, USA.: SAS Institute Inc. ; 2003.
15. Mota Rojas D, Alarcón A, Huertas SM, Guerrero-Legarreta I. Músculo oscuro, firme y seco en bovinos: mecanismos involucrados. In: Mota Rojas D, Huertas SM, Guerrero Legarreta I, Trujillo ME, editors. Bienestar Animal: Productividad y Calidad de la Carne. México: Elsevier; 2012. p. 477-95.
16. Ngoka DA, Froning GW. Effect of Free Struggle and Preslaughter Excitement on Color of Turkey Breast Muscles. Poultry Science. 1982;61(11):2291-3. http://dx.doi.org/10.3382/ps.0612291.
17. Warriss PD, Knowles TG, Brown SN, Edwards JE, Kettlewell PJ, Mitchell MA et al. Effects of lairage time on body temperature and glycogen reserves of broiler chickens held in transport modules. Veterinary Record. 1999;145(8):218-22. http://dx.doi.org/10.1136/vr.145.8.218.
18. Nannan J, Tong X, Xinglian X. Effects of pre‐slaughter showering and ventilation on stress, meat quality and metabolite concentrations of broilers in summer. Animal Science Journal. 2016;87:293-8. https://doi.org/10.1111/asj.12419
19. Surmei E, Usturoi MG. Studies on freshness of refrigerated poultry meat. Analele Universităţii din Oradea. 2012:115-21. http://protmed.uoradea.ro/facultate/anale/ecotox_zooteh_ind_alim/2012A/imapa/21.ELENA%20SURMEI.pdf
20. Zhu X-s, Xu X-l, Min H-h, Zhou G-h. Occurrence and Characterization of Pale, Soft, Exudative-Like Broiler Muscle Commercially Produced in China. Journal of Integrative Agriculture. 2012;11(8):1384-90. https://doi.org/10.1016/S2095-3119(12)60137-3.
21. Debut M, Berri C, Baeza E, Sellier N, Arnould C, Guemene D et al. Variation of chicken technological meat quality in relation to genotype and preslaughter stress conditions. Poultry Science. 2003;82(12):1829-38. http://dx.doi.org/10.1093/ps/82.12.1829.
22. Bianchi M, Petracci M, Cavani C. The influence of genotype, market live weight, transportation, and holding conditions prior to slaughter on broiler breast meat color. Poultry Science. 2006;85(1):123-8. https://doi.org/10.1093/ps/85.1.123
23. Lázaro CA, Conte-Júnior CA, Monteiro MLG, Canto ACVS, Costa-Lima BRC, Mano SB et al. Effects of ultraviolet light on biogenic amines and other quality indicators of chicken meat during refrigerated storage. Poultry Science. 2014;93(9):2304-13. http://dx.doi.org/10.3382/ps.2013-03642.
24. Qiao M, Fletcher DL, Northcutt JK, Smith DP. The Relationship Between Raw Broiler Breast Meat Color and Composition. Poultry Science. 2002;81(3):422-7. http://dx.doi.org/10.1093/ps/81.3.422.
25. Garcia RG, Freitas LWd, Schwingel AW, Farias RM, Caldara FR, Gabriel AMA et al. Incidence and physical properties of PSE chicken meat in a commercial processing plant. Revista Brasileira de Ciência Avícola. 2010;12(4):233-7. http://dx.doi.org/10.1590/S1516-635X2010000400003
26. Lázaro CA, Conte-Júnior CA, Canto AC, Monteiro MLG, Costa-Lima B, Cruz AGd et al. Biogenic amines as bacterial quality indicators in different poultry meat species. LWT - Food Science and Technology. 2015;60(1):15-21. http://dx.doi.org/10.1016/j.lwt.2014.09.025.
27. Balamatsia CC, Paleologos EK, Kontominas MG, Savvaidis IN. Correlation between microbial flora, sensory changes and biogenic amines formation in fresh chicken meat stored aerobically or under modified atmosphere packaging at 4 degrees C: possible role of biogenic amines as spoilage indicators. Anton Leeuw Int J G. 2006;89(1):9-17. https://doi.org/10.1007/s10482-005-9003-4
28. Sander JE, Cai T, Dale N, Bennett LW. Development of Biogenic Amines During Fermentation of Poultry Carcasses. The Journal of Applied Poultry Research. 1996;5(2):161-6. http://dx.doi.org/10.1093/japr/5.2.161.
29. Kozová M, Kalač P, Pelikánová T. Contents of biologically active polyamines in chicken meat, liver, heart and skin after slaughter and their changes during meat storage and cooking. Food Chemistry. 2009;116(2):419-25. http://dx.doi.org/10.1016/j.foodchem.2009.02.057.
30. Rokka M, Eerola S, Smolander M, Alakomi H-L, Ahvenainen R. Monitoring of the quality of modified atmosphere packaged broiler chicken cuts stored in different temperature conditions: B. Biogenic amines as quality-indicating metabolites. Food Control. 2004;15(8):601-7. https://doi.org/10.1016/j.foodcont.2003.10.002
31. Silva CMG, Gloria MBA. Bioactive amines in chicken breast and thigh after slaughter and during storage at 4+/-1 °C and in chicken-based meat products. Food Chemistry. 2002;78(2):241-8. https://doi.org/10.1016/S0308-8146(01)00404-6
32. Vieira FMC, Silva IJO, Barbosa Filho JAD, Vieira AMC, Broom DM. Preslaughter mortality of broilers in relation to lairage and season in a subtropical climate. Poultry Science. 2011;90(10):2127-33. http://dx.doi.org/10.3382/ps.2010-01170.
33. Dave D, Ghaly AE. Meat Spoilage Mechanisms and Preservation Techniques: A Critical Review. American Journal of Agricultural and Biological Science 2011;6(4):486-510. http://dx.doi.org/10.3844/ajabssp.2011.486.510
34. Özogul Y, Özogul F. Effects of slaughtering methods on sensory, chemical and microbiological quality of rainbow trout (Onchorynchus mykiss) stored in ice and MAP. Eur Food Res Technol. 2004;219(3):211-6. http://dx.doi.org/10.1007/s00217-004-0951-0.
35. Cruz-Monterrosa RG, Reséndiz-Cruz V, Rayas-Amor AA, López M, Miranda-de la Lama GC. Bruises in beef cattle at slaughter in Mexico: implications on quality, safety and shelf life of the meat. Tropical Animal Health and Production. 2017;49(1):145-52. http://dx.doi.org/10.1007/s11250-016-1173-8